
Impact of sepsis on Eastern Cooperative Oncology Group performance status among fully ambulatory patients: a prospective nationwide multicenter cohort
Highlight box
Key findings
• One in five septic patients who were fully ambulatory before sepsis were not functionally independent at hospital discharge.
• The adequacy of empirical antibiotics may improve the functional status in such patients.
What is known and what is new?
• Sepsis survivors often showed incomplete recovery of their functional status and health-related quality of life after hospital discharge in previous studies.
• In the present study, one in five septic patients who were fully ambulatory before sepsis did not recover their functional independence at hospital discharge. Incomplete functional recovery was also seen in a substantial proportion of younger patients (<65 years), those with low comorbidities, and those without septic shock.
What is the implication, and what should change now?
• Multidisciplinary efforts, including increased compliance with adequate empirical antibiotics, may improve the functional outcomes in sepsis patients, especially in those who are fully ambulatory.
Introduction
Sepsis is a clinical syndrome caused by a dysregulated host inflammatory response to infections and is associated with organ dysfunction (1). It is considered a medical emergency due to high associated mortality and complication rates despite appropriate treatment (2-5).
Although the survival rates have increased with early diagnosis and treatment, sepsis survivors often do not recover their prior functional status (6). A prospective study of septic patients found incomplete recovery of health-related quality of life at 6 months after intensive care unit (ICU) discharge (7). In a retrospective study, 63% of patients with severe sepsis or septic shock had a good functional status before sepsis; however, only 36% of sepsis survivors had a good functional status at 1 year after hospital discharge (8). Importantly, sepsis can occur in healthy populations who are fully ambulatory and engaged in social activities. The consequent cognitive and physical dysfunction and increased dependency may cause serious economic problems for patients and their families (9). However, there has been limited clinical research on the impact of sepsis on functional status in healthy populations.
Eastern Cooperative Oncology Group performance status (ECOG PS), an indicator of frailty, indicates a patient’s ability to perform activities of daily living (10). Although the scale was validated for oncological patients (10-12), it has also been useful for patients with various conditions, including critically ill conditions where ECOG PS was associated with treatment outcomes (13-15). Therefore, using data from a nationwide, prospective sepsis registry by the Korean Sepsis Alliance (KSA), we evaluated the change in ECOG PS and the factors associated with its deterioration during the treatment of sepsis in patients who were fully ambulatory prior to sepsis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-405/rc).
Methods
Study population
We used sepsis data from an ongoing nationwide, multicenter cohort operated by the KSA. In total, 19 tertiary or university-affiliated hospitals (20 ICUs) in South Korea that run educational programs on sepsis bundles participated in this study. Regular audits were conducted by the KSA research committee members to verify the data quality (16). This is a retrospective analysis of data that were prospectively collected over a 17-month period between August 2019 and December 2020. We screened consecutive adult patients (aged ≥19 years) who were diagnosed with sepsis in emergency departments (EDs) or general wards. The third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) were used to diagnose sepsis (17).
To evaluate the level of functioning of patients in terms of their ability to perform their activities of daily life, data on ECOG PS (score: 0 to 5) were collected at the time of sepsis diagnosis (i.e., PS before sepsis diagnosis) and at hospital discharge (i.e., PS after sepsis treatment) by trained study coordinators; data were confirmed by the study investigators of each hospital (10). We defined ECOG of 0 or 1 as good PS and ECOG ≥2 as poor PS (18,19). Patients with good ECOG PS (i.e., 0 and 1) before sepsis diagnosis were ultimately included in the study. Patients with do-not-resuscitate (DNR) orders, missing values, or disability before sepsis diagnosis (ECOG PS ≥2), and those who died in hospitals were excluded.
Data collection
The study coordinators at each participating center prospectively collected data and entered the information into a web-based database system (http://sepsis.crf.kr/). We collected the following data: demographic data, including age, sex, body mass index (BMI); underlying comorbidities, including Charlson comorbidity index (CCI); disease severity [sequential organ failure assessment (SOFA) score]; physiological and laboratory measurements; infection source and type (i.e., community- or hospital-acquired infections); presence of multidrug-resistant (MDR) pathogens in cases of culture-positive patients; sepsis treatment, including completion of the 3 h sepsis bundle, appropriateness of empirical antibiotic therapy, transfusions, use of steroids and vasopressors, mechanical ventilation (MV), and continuous renal replacement therapy (CRRT); and lengths of stay in the ICU and hospital
Community-acquired sepsis was defined as infection acquired in a community setting, whereas hospital-acquired sepsis was defined as infection that developed 48 h after hospitalization. The “time zero” for community-acquired sepsis was defined as the time of triage in the ED, whereas that for hospital-acquired sepsis was when the rapid response team diagnosed sepsis in the general ward (20,21). The appropriateness of empirical antibiotics was determined according to the drug susceptibility test results or according to the relevant guidelines (22,23). MDR pathogens were defined as those resistant to agents from at least three antimicrobial categories (24).
Data analysis and statistical methods
Primary outcomes were changes in ECOG PS between time zero (i.e., before sepsis) and hospital discharge, and the proportion of patients with poor ECOG PS (≥2) at hospital discharge. The secondary outcomes were as follows: risk factors for poor ECOG PS at hospital discharge, which were investigated using multivariable analysis; the prevalence of poor ECOG PS according to age (<65 vs. ≥65 years), history of cancer, underlying comorbidity (i.e., CCI of ≤2 vs. >2), and septic shock; and the different prevalence of poor ECOG PS by the coronavirus disease 2019 (COVID-19) pandemic (pre- vs. intra-COVID-19 pandemic).
Descriptive analyses were used to summarize the characteristics of patients. Continuous data are expressed as means ± standard deviations or median [interquartile ranges (IQRs), 25th–75th percentiles], whereas categorical data are presented as frequency (percentage). The χ2 test or Fisher’s exact test was used to analyze categorical variables, whereas the Student’s t-test and Mann-Whitney U test were used for continuous variables. Logistic regression analysis was performed using covariates with P<0.10 in univariable analysis to identify factors associated with poor ECOG PS at the time of hospital discharge. Initially, a total of sixteen variables (P<0.10) were included in the model; age, CCI, chronic heart disease, solid cancer, immunocompromised, SOFA score, MDR pathogens, lactate, septic shock, steroid therapy, use of vasopressors, inappropriate empirical antibiotics, MV, transfusions, CRRT, and ICU admission. A backward stepwise selection method based on the likelihood ratio was used, and eight variables finally remained in the multivariable model. For the model calibration, Hosmer-Lemeshow test was used (chi-square =6.943, P=0.543). All tests were two-sided, and P<0.05 was considered to indicate statistical significance. The statistical analyses were performed using R software (version 4.3.0; R Foundation for Statistical Computing; Vienna, Austria).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review boards of all participating hospitals, including the Hallym University Institutional Review Board (approval No. 2018-09-004). Because this was an observational study with no interventions, the decision to obtain written informed consent from patients or their legal surrogates was at the discretion of the ethics committees of the participating hospitals.
Results
Study population
Of the 7,113 septic patients in the KSA cohort, 2,968 were excluded due to DNR order (n=2,428) or missing values (n=540), and 4,145 were initially included. Of them, 1,880 (45.4%) patients were previously fully ambulatory (i.e., ECOG PS 0 or 1), and after the exclusion of patients who eventually died in the hospital (n=145), 1,735 were finally included in the analysis (Figure 1). The mean age was 66.1±13.5 years, and 60.3% were males (Table 1). The median lactate level of all 1,735 patients was 2.3 (1.4–3.8) mmol/L, and the proportion of patients with septic shock was 19.0%. The most common site of sepsis origin was the abdomen (38.4%), followed by the lungs (30.8%) and urinary tract (16.6%). The median SOFA score was 5.0 (3.0–7.0), and the median CCI was 5.0 (3.0–6.0). Bacteremia was found in 19.6% (340/1,735) of patients, and hospital-acquired infections accounted for 31.2% of infections. The baseline laboratory parameters are presented in Table S1.
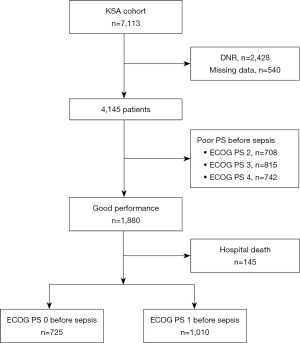
Table 1
| Variables | Total (n=1,735) | Good ECOG PS† (n=1,359) | Poor ECOG PS‡ (n=376) | P value |
|---|---|---|---|---|
| Age, years | 66.1±13.5 | 65.5±13.7 | 68.4±12.2 | <0.001 |
| Sex, male | 1,046 (60.3) | 817 (60.1) | 229 (60.9) | 0.829 |
| BMI, kg/m2 | 22.9±4.0 | 23.0±4.0 | 22.8±4.1 | 0.425 |
| Charlson comorbidity index | 5.0 (3.0–6.0) | 5.0 (3.0–6.0) | 5.0 (4.0–7.0) | <0.001 |
| ECOG 0/ECOG 1 before sepsis | 725/1,010 | 568/791 | 157/219 | 0.989 |
| Underlying comorbidities | ||||
| Diabetes | 642 (37.0) | 494 (36.4) | 148 (39.4) | 0.313 |
| Chronic heart disease | 450 (25.9) | 393 (28.9) | 57 (15.2) | <0.001 |
| Chronic lung disease | 276 (15.9) | 223 (16.4) | 53 (14.1) | 0.315 |
| Chronic liver disease | 211 (12.2) | 161 (11.8) | 50 (13.3) | 0.501 |
| Chronic kidney disease | 252 (14.5) | 196 (14.4) | 56 (14.9) | 0.883 |
| Cerebrovascular accident | 196 (11.3) | 148 (10.9) | 48 (12.8) | 0.355 |
| Solid cancer | 590 (34.0) | 431 (31.7) | 159 (42.3) | <0.001 |
| Hematologic disease | 101 (5.8) | 74 (5.4) | 27 (7.2) | 0.251 |
| Immunocompromised | 93 (5.4) | 61 (4.5) | 32 (8.5) | 0.003 |
| Origin of sepsis | ||||
| Abdomen | 667 (38.4) | 528 (38.9) | 139 (37.0) | 0.545 |
| Catheter-associated infection | 11 (0.6) | 7 (0.5) | 4 (1.1) | 0.267 |
| Neurology | 142 (8.2) | 106 (7.8) | 36 (9.6) | 0.315 |
| Pulmonary | 534 (30.8) | 410 (30.2) | 124 (33.0) | 0.326 |
| Skin and soft tissue | 85 (4.9) | 64 (4.7) | 21 (5.6) | 0.575 |
| Urinary | 288 (16.6) | 238 (17.5) | 50 (13.3) | 0.062 |
| Sepsis without clear origins | 8 (0.5) | 6 (0.4) | 2 (0.5) | 0.686 |
| Initial SOFA score | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 6.0 (4.0–8.0) | <0.001 |
| Initial lactate, mmol/L | 2.3 (1.4–3.8) | 2.2 (1.4–3.6) | 2.6 (1.6–4.6) | <0.001 |
| HAI | 542 (31.2) | 423 (31.1) | 119 (31.6) | 0.896 |
| Septic shock | 329 (19.0) | 233 (17.1) | 96 (25.5) | <0.001 |
| Pathogen identified | 1,023 (59.0) | 792 (58.3) | 231 (61.4) | 0.297 |
| Bacteria/virus/fungus/others | 932/35/39/17 | 722/29/27/14 | 210/6/12/3 | 0.455 |
| Bacteremia§ | 340 (36.5) | 272 (37.7) | 68 (32.4) | 0.161 |
| MDR pathogens§ | 300 (32.2) | 220 (30.5) | 80 (38.1) | 0.037 |
Values are presented as mean ± SD, median (interquartile range), or number (%). †, ECOG of 0 or 1 at hospital discharge. ‡, ECOG of 2 or more at hospital discharge. §, among patients with bacterial infections. ECOG PS, Eastern Cooperative Oncology Group performance status; BMI, body mass index; SOFA, sequential organ failure assessment; HAI, hospital-acquired infection; MDR, multidrug-resistant; SD, standard deviation.
Changes in ECOG performance status
Of the 1,735 patients with good ECOG PS (0 or 1) before sepsis diagnosis, the PS deteriorated at hospital discharge in 514 (29.6%) patients, and 21.7% of each ECOG PS group (0 or 1) had poor ECOG PS (≥2) at hospital discharge (Figure 2 and Table S2).
Comparison of patients with good and poor ECOG PS at hospital discharge
As shown in Tables 1,2, there were significant differences in the baseline characteristics and treatments between the two ECOG PS groups. Patients with good ECOG PS at hospital discharge (n=1,359) were younger, had lower CCI scores compared to those with poor ECOG PS (n=376). The former group also showed a lower disease severity at sepsis diagnosis, reflected by a lower SOFA score and lactate level, and less frequently received treatments with transfusions, MV, or CRRT compared to the latter group. However, more appropriate empirical antibiotics were administered among those with good ECOG PS at hospital discharge (94.6% vs. 89.6%, P<0.001).
Table 2
| Variables | Total (n=1,735) | Good ECOG PS† (n=1,359) | Poor ECOG PS‡ (n=376) | P value |
|---|---|---|---|---|
| Treatments | ||||
| Steroid therapy | 296 (17.1) | 221 (16.3) | 75 (19.9) | 0.109 |
| Vasopressors | 439 (25.3) | 317 (23.3) | 122 (32.4) | <0.001 |
| Antibiotic adequacy | 1,623 (93.5) | 1,286 (94.6) | 337 (89.6) | <0.001 |
| Mechanical ventilation | 279 (16.1) | 162 (11.9) | 117 (31.1) | <0.001 |
| Transfusion | 300 (17.3) | 204 (15.0) | 96 (25.5) | <0.001 |
| CRRT | 112 (6.5) | 69 (5.1) | 43 (11.4) | <0.001 |
| 3-h bundle compliance | 922 (53.1) | 721 (53.1) | 201 (53.5) | 0.936 |
| ICU admission | 744 (42.9) | 546 (40.2) | 198 (52.7) | <0.001 |
| Hospital outcomes | ||||
| Length of hospital stay, days | 13.0 (7.0–22.0) | 12.0 (7.0–19.0) | 18.0 (7.5–36.0) | <0.001 |
| Length of ICU stay, days§ | 4.0 (2.0–8.0) | 4.0 (2.0–6.0) | 7.0 (3.0–14.3) | <0.001 |
Values are presented as number (%) or median (interquartile range). †, ECOG of 0 or 1 at hospital discharge. ‡, ECOG of 2 or more at hospital discharge. §, among 744 patients who were admitted to the ICU. ECOG PS, Eastern Cooperative Oncology Group performance status; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
Prevalence of poor ECOG PS by subgroups
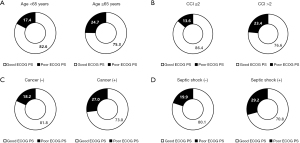
A substantial proportion of patients younger than 65 years [17.4% (126/724)], those with low comorbidities [i.e., CCI ≤2; 13.6% (41/301)], those without a history of cancer [18.2% (193/1,058)], and those without septic shock [19.9% (280/1,406)] had poor ECOG PS at hospital discharge (Figure 3). The frequency of poor ECOG PS increased with increasing baseline SOFA score quartiles and lactate levels (P<0.001 and P=0.011, respectively; Figure 4).

Multivariable analysis of risk factors for disability at hospital discharge
Initially, 16 variables with P<0.10 in the univariable analysis (Table S3) were incorporated into the logistic regression analysis. Ultimately, eight variables remained in the final model, and among them, age, chronic heart disease, solid cancer, immunocompromised condition, SOFA score, use of inappropriate empirical antibiotics, and MV were significantly associated with poor ECOG PS at hospital discharge (Table 3). Especially, the use of inappropriate empirical antibiotics [odds ratio (OR): 1.786; 95% confidence interval (CI): 1.151–2.771] was the only modifiable factor.
Table 3
| Variables | P value | OR | 95% CI |
|---|---|---|---|
| Age, years | <0.001 | 1.031 | 1.020–1.042 |
| Chronic heart disease | <0.001 | 0.403 | 0.289–0.560 |
| Solid cancer | 0.001 | 1.514 | 1.177–1.948 |
| Immunocompromised | 0.002 | 2.130 | 1.315–3.449 |
| SOFA score | 0.001 | 1.080 | 1.032–1.130 |
| Lactate | 0.074 | 1.042 | 0.996–1.091 |
| Inappropriate antibiotics | 0.010 | 1.786 | 1.151–2.771 |
| MV | <0.001 | 3.030 | 2.258–4.064 |
†, Sixteen variables with a P value <0.1 in univariable analyses were initially included in the model: age, CCI, chronic heart disease, solid cancer, immunocompromised, SOFA score, MDR pathogens, lactate, septic shock, steroid therapy, use of vasopressors, inappropriate antibiotics, MV, transfusion, CRRT, and ICU admission. ‡, Hosmer-Lemeshow test: chi-square =6.943, df =8, P value =0.543. ECOG PS, Eastern Cooperative Oncology Group performance status; OR, odds ratio; CI, confidence interval; SOFA, sequential organ failure assessment; MV, mechanical ventilation. CCI, Charlson Comorbidity Index; MDR, multi-drug resistant; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
Prevalence of poor ECOG PS according to the COVID-19 pandemic period
The prevalence rates of poor ECOG PS at hospital discharge were significantly different between the pre- and intra-COVID-19 pandemic periods [102 (18.2%) vs. 274 (23.3%), P=0.017; Figure S1]. When included in the multivariable model, the intra-COVID-19 pandemic period was associated with increased risk of poor ECOG PS at hospital discharge (OR: 1.411; 95% CI: 1.078–1.860) (Table S4).
Discussion
In this prospective sepsis cohort, we found that one in five septic patients with good functional status (ECOG PS of 0 or 1) before sepsis did not recover their previous functional status at hospital discharge. Second, incomplete functional recovery was also found in a substantial proportion of young patients (aged <65 years), patients with low comorbidities, and patients without septic shock. Third, age, solid cancer, immunocompromised condition, SOFA score, use of inappropriate empirical antibiotics, and MV were significant predictors for poor ECOG PS at hospital discharge. In particular, the identification of the use of inappropriate empirical antibiotics as a risk factor for poor ECOG was remarkable, which may be modifiable by performance improvement programs in clinical practice.
Sepsis is a life-threatening organ dysfunction by a dysregulated host response to infections and frequently causes long-term consequences (17). “Post-sepsis syndrome”, or “post-intensive care syndrome (PICS)”, is characterized by neurocognitive impairment, functional disability, psychological deficits, and worsening medical condition (6,25). Although the mechanism is multifactorial, systemic inflammatory processes, leading to altered muscle integrity and function, may play a role in the development of physical impairment (26,27). In previous studies, old age, low BMI, disease severity, and prolonged MV were associated with functional impairment after critical illnesses (27,28). The increasing severity of sepsis or organ failure was also linked to worsening functional status (29). Similarly, in the present study, we found that old age, comorbidities (solid cancer and immunocompromised), and severity of illness (initial SOFA and MV) were associated with poor ECOG PS at hospital discharge, which reflects the adverse effects of malnutrition, severe underlying illness, or systemic inflammation on patients’ functional outcomes. However, contrary to previous studies, we could not find any associations of low BMI with poor ECOG PS, and unexpectedly, a history of chronic heart disease showed a protective effect. These can be explained by different populations and low statistical power of our study. However, the exclusion of the non-survivors might have affected the results; BMI was lower, and a history of chronic heart disease was more frequent in the non-survivors than in the survivors (data not shown).
Unlike previous studies that reported functional outcomes over the long-term period (e.g., 6 months or 1 year after discharge), our study focused on the functional status at hospital discharge. Although this can be one of the limitations of our study and attributable to a problem of using registry data, several studies also reported functional outcomes at the time of hospital discharge. In a retrospective study in the United States, 29.3% of non-surgical patients had functional decline between ICU admission and hospital discharge (30). Another single-center study reported 42.5% of sepsis survivors who had hospital-acquired functional decline. In this study, lower pre-hospital functional status and longer time to initial ambulation were associated with the functional decline (31). However, in the present study, we found that a significant proportion of young patients (aged <65 years), those with low comorbidities or no history of cancer, and those without septic shock had poor ECOG PS at hospital discharge, implying that they may not be able to return to their previous level of activity immediately. This can be linked to socioeconomic burden of sepsis. Experts say that lower productivity and indirect medical costs after hospital discharge, rather than initial hospitalization costs, account for the majority of the total costs (32). Particularly, education levels, which are associated with a better support system and recovery program, have been found to be associated with functional status post-ICU discharge (33). Therefore, these should be considered important when assessing socioeconomic burden of sepsis.
Another important finding is that the use of inappropriate empirical antibiotics was significantly associated with poor ECOG PS at hospital discharge. Previously, the appropriateness of empirical antibiotics was known to be important for improving patient outcomes in sepsis (34). However, our results suggest that it may also improve the ECOG PS, not just decrease in-hospital mortality. This is likely to be associated with faster recovery or shorter hospital stay (35), but the exact mechanism remains to be established in future studies. Notably, the rate of appropriate empirical antibiotics was high in our cohort (93.5%), making it hard to expect a further improvement in clinical practice, However, on the other hand, the prevention of the inadequacy of empirical antibiotics may have a greater effect on a system with lower antibiotic compliance rate.
Interestingly, in another multivariable model in our study (Table S4), the COVID-19 pandemic period was associated with increased risk of poor ECOG PS at hospital discharge. which was consistent with previous studies reporting an increased burden of severe COVID-19 in terms of functional outcomes (36,37). However, in the model, inappropriate empirical antibiotics, as well as other factors (i.e., age, immunocompromised, SOFA score, and MV), were still significant risk factors for poor ECOG PS.
In clinical practice, it is crucial to accurately assess the PS of critically ill patients to inform clinical decisions. ECOG is simple and easily understood by nurses and physicians, which can enhance the consistency in recordings and lead to less variation among investigators (11,38). However, ECOG was developed for oncological patients, and there are other well-validated scales for functional status for non-oncological patients, such as Functional Independence Measure (39), the World Health Organization's Disability Assessment Schedule 2.0 (WHODAS II) (40), and Barthel Index (41). Unfortunately, we could not use these scales, representing a limitation of our study. However, several studies reported the usefulness of ECOG PS for predicting outcomes in critically ill patients (14,15). And, in our study, the data on ECOG were collected by trained study coordinators of each hospital and confirmed again by the investigators, which may strengthen the reliability of our data.
Our study had several limitations. First, our results may be influenced by the inherent selection bias in this observational study. However, the data were prospectively collected from all consecutive patients diagnosed with sepsis at 19 hospitals in South Korea. Second, as aforementioned, ECOG was originally developed and validated for oncological patients, and we were not able to formally evaluate the inter-rater consistency or reliability of the ECOG scores. Third, data on long-term outcomes, such as 6-month and 1-year mortalities and quality of life, were not evaluated. In particular, the cognitive and psychological functions were not investigated. These data would have provided additional information regarding sepsis burden. Fourth, the study was conducted in a single country, thus limiting the generalizability of our findings. Particularly, abdominal infections were the most frequent source of sepsis, which was different from those in other sepsis studies (42,43). This may be associated with the exclusion of the patients who have died [that is, the proportion of pneumonia was 40.0% (58/145 patients) of the non-survivors]. Hence, caution should be taken when interpreting our data. Finally, although early rehabilitation programs in the ICU can improve the PS outcomes, this was not investigated. However, despite these limitations, a distinct feature of our study is that we focused on relatively healthy patients who lived independently before sepsis. We also identified a modifiable factor that affects the PS outcomes in these patients. Therefore, our results may aid the estimation of the sepsis burden and contribute to improving sepsis outcomes.
Conclusions
One in five septic patients who were fully ambulatory before sepsis were not functionally independent at hospital discharge. In particular, incomplete functional recovery was also found in a substantial proportion of younger patients, those with low comorbidities, and those without septic shock. However, the inadequacy of empirical antibiotics was a risk factor for poor ECOG PS; its prevention may improve the functional outcomes. Further studies are warranted to elucidate the sepsis burden more accurately and to identify additional modifiable factors of functional outcomes.
Acknowledgments
The following persons and institutions participated in the Korean Sepsis Alliance (KSA): Steering Committee —Chae-Man Lim (Chair), Sang-Bum Hong, Dong Kyu Oh, Gee Young Suh, Kyeongman Jeon, Ryoung-Eun Ko, Young-Jae Cho, Yeon Joo Lee, Sung Yoon Lim, Sunghoon Park; Participated Persons and Centers—Kangwon National University Hospital—Jeongwon Heo; Korea University Anam Hospital—Jae-myeong Lee; Daegu Catholic University Hospital—Kyung Chan Kim; Seoul National University Bundang Hospital—Yeon Joo Lee; Inje University Sanggye Paik Hospital—Youjin Chang; Samsung Medical Center—Kyeongman Jeon; Seoul National University Hospital—Sang-Min Lee; Asan Medical Center—Chae-Man Lim, Suk-Kyung Hong; Pusan National University Yangsan Hospital—Woo Hyun Cho; Chonnam National University Hospital—Sang Hyun Kwak; Jeonbuk National University Hospital—Heung Bum Lee; Ulsan University Hospital—Jong-Joon Ahn; Jeju National University Hospital—Gil Myeong Seong; Chungnam National University Hospital—Song-I Lee; Hallym University Sacred Heart Hospital—Sunghoon Park; Hanyang University Guri Hospital—Tai Sun Park; Severance Hospital—Su Hwan Lee; Yeungnam University Medical Center—Eun Young Choi; Chungnam National University Sejong Hospital—Jae Young Moon.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-405/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-405/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-405/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-405/coif). CML reports research grants from the Research Program funded by the Korea Disease Control and Prevention Agency (fund codes 2019E280500, 2020E280700, and 2021-10-026) and the Korean Society of Critical Care Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018;392:75-87. [Crossref] [PubMed]
- Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015;15:581-614. [Crossref] [PubMed]
- Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA 2018;319:62-75. [Crossref] [PubMed]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. [Crossref] [PubMed]
- Zhang Z, Smischney NJ, Zhang H, et al. AME evidence series 001-The Society for Translational Medicine: clinical practice guidelines for diagnosis and early identification of sepsis in the hospital. J Thorac Dis 2016;8:2654-65. [Crossref] [PubMed]
- Mostel Z, Perl A, Marck M, et al. Post-sepsis syndrome - an evolving entity that afflicts survivors of sepsis. Mol Med 2019;26:6. [Crossref] [PubMed]
- Hofhuis JG, Spronk PE, van Stel HF, et al. The impact of critical illness on perceived health-related quality of life during ICU treatment, hospital stay, and after hospital discharge: a long-term follow-up study. Chest 2008;133:377-85. [Crossref] [PubMed]
- Al Khalaf MS, Al Ehnidi FH, Al-Dorzi HM, et al. Determinants of functional status among survivors of severe sepsis and septic shock: One-year follow-up. Ann Thorac Med 2015;10:132-6. [Crossref] [PubMed]
- Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787-94. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Azam F, Latif MF, Farooq A, et al. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep Oncol 2019;12:728-36. [Crossref] [PubMed]
- Kato Y, Hosomi Y, Watanabe K, et al. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Dis 2019;11:2350-60. [Crossref] [PubMed]
- Iwasaki M, Ishikawa M, Namizato D, et al. Worse ECOG-PS Is Associated with Increased 30-Day Mortality among Adults Older than 90 Years Undergoing Non-Cardiac Surgery: A Single-Center Retrospective Study. J Nippon Med Sch 2022;89:295-300. [Crossref] [PubMed]
- Zampieri FG, Bozza FA, Moralez GM, et al. The effects of performance status one week before hospital admission on the outcomes of critically ill patients. Intensive Care Med 2017;43:39-47. [Crossref] [PubMed]
- Park CM, Koh Y, Jeon K, et al. Impact of Eastern Cooperative Oncology Group Performance Status on hospital mortality in critically ill patients. J Crit Care 2014;29:409-13. [Crossref] [PubMed]
- Jeon K, Na SJ, Oh DK, et al. Characteristics, management and clinical outcomes of patients with sepsis: a multicenter cohort study in Korea. Acute Crit Care 2019;34:179-91. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Kim HS, Ryu MH, Zang DY, et al. Phase II study of oxaliplatin, irinotecan and S-1 therapy in patients with advanced gastric cancer: the Korean Cancer Study Group ST14-11. Gastric Cancer 2018;21:802-10. [Crossref] [PubMed]
- Catalano M, Roviello G, Conca R, et al. Clinical Outcomes and Safety of Patients Treated with NAb-Paclitaxel Plus Gemcitabine in Metastatic Pancreatic Cancer: The NAPA Study. Curr Cancer Drug Targets 2020;20:887-95. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552. [Crossref] [PubMed]
- Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 Update. Crit Care Med 2018;46:997-1000. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27-72. [Crossref] [PubMed]
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81. [Crossref] [PubMed]
- Huang CY, Daniels R, Lembo A, et al. Life after sepsis: an international survey of survivors to understand the post-sepsis syndrome. Int J Qual Health Care 2019;31:191-8. [Crossref] [PubMed]
- Witteveen E, Wieske L, van der Poll T, et al. Increased Early Systemic Inflammation in ICU-Acquired Weakness; A Prospective Observational Cohort Study. Crit Care Med 2017;45:972-9. [Crossref] [PubMed]
- Rengel KF, Hayhurst CJ, Pandharipande PP, et al. Long-term Cognitive and Functional Impairments After Critical Illness. Anesth Analg 2019;128:772-80. [Crossref] [PubMed]
- Musheyev B, Borg L, Janowicz R, et al. Functional status of mechanically ventilated COVID-19 survivors at ICU and hospital discharge. J Intensive Care 2021;9:31. [Crossref] [PubMed]
- Rai R, Singh R, Azim A, et al. Impact of Critical Illness on Quality of Life after Intensive Care Unit Discharge. Indian J Crit Care Med 2020;24:299-306. [Crossref] [PubMed]
- Ingraham NE, Vakayil V, Pendleton KM, et al. National Trends and Variation of Functional Status Deterioration in the Medically Critically Ill. Crit Care Med 2020;48:1556-64. [Crossref] [PubMed]
- Takahashi Y, Morisawa T, Okamoto H, et al. Prevalence and predictors of hospital-acquired functional decline in patients with sepsis admitted to the intensive care unit. Int J Rehabil Res 2021;44:307-13. [Crossref] [PubMed]
- Tiru B, DiNino EK, Orenstein A, et al. The Economic and Humanistic Burden of Severe Sepsis. Pharmacoeconomics 2015;33:925-37. [Crossref] [PubMed]
- Marra A, Pandharipande PP, Girard TD, et al. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit Care Med 2018;46:1393-401. [Crossref] [PubMed]
- Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Crit Care 2015;19:302. [Crossref] [PubMed]
- González-Del Castillo J, Domínguez-Bernal C, Gutiérrez-Martín MC, et al. Effect of the inadequacy of antibiotic therapy in the Emergency Department on hospital stays. Enferm Infecc Microbiol Clin 2017;35:208-13. [PubMed]
- Stripari Schujmann D, Claudia Lunardi A, Neri Peso C, et al. Functional Recovery Groups in Critically Ill COVID-19 Patients and Their Associated Factors: From ICU to Hospital Discharge. Crit Care Med 2022;50:1799-808. [Crossref] [PubMed]
- Rousseau AF, Minguet P, Colson C, et al. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann Intensive Care 2021;11:118. [Crossref] [PubMed]
- Simcock R, Wright J. Beyond Performance Status. Clin Oncol (R Coll Radiol) 2020;32:553-61. [Crossref] [PubMed]
- Herridge MS, Chu LM, Matte A, et al. The RECOVER Program: Disability Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. Am J Respir Crit Care Med 2016;194:831-44. [Crossref] [PubMed]
- Hodgson CL, Udy AA, Bailey M, et al. The impact of disability in survivors of critical illness. Intensive Care Med 2017;43:992-1001. [Crossref] [PubMed]
- Wilson ME, Barwise A, Heise KJ, et al. Long-Term Return to Functional Baseline After Mechanical Ventilation in the ICU. Crit Care Med 2018;46:562-9. [Crossref] [PubMed]
- Martin CM, Priestap F, Fisher H, et al. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med 2009;37:81-8. [Crossref] [PubMed]
- Phua J, Koh Y, Du B, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ 2011;342:d3245. [Crossref] [PubMed]



