
The impact of postoperative cerebrospinal fluid drainage on neurological improvement following thoracic aortic and thoracoabdominal aortic surgery
Highlight box
Key findings
• Postoperative cerebrospinal fluid drainage (CSFD) improved neurological prognosis after thoracic aortic and thoracoabdominal aortic surgery.
What is known and what is new?
• It is unclear how effective postoperative CSFD for thoracic aortic and thoracoabdominal aortic surgery is in terms of neurological
• A patient who developed postoperative paraplegia/paraparesis showed neurological improvement with postoperative CSFD
What is the implication, and what should change now?
• Postoperative CSFD showed neurological improvement, while a group of patients did not respond at all. Management of this group of patients needs to be considered.
Introduction
Paraplegia is a major complication in the treatment of thoracic and thoracoabdominal aortic aneurysms. The incidence of paraplegia following thoracic descending aortic aneurysm repair has been reported to be 10–25% (1).
Paraplegia severely compromises the patient’s quality of life, and often leads to death from nonvascular causes (2). To avoid paraplegia, surgeons have used a variety of techniques, including preoperative identification of the Adamkiewicz artery (AKA), intraoperative detection of decreased spinal cord perfusion using intercostal artery perfusion, intercostal artery reconstruction, and motor evoked potentials (MEP), as well as postoperative methods for increasing oxygen delivery to the spinal cord based on COPS [cerebrospinal fluid drainage (CSFD), oxygen delivery, and patients status (COPS)] treatment. Although the incidence of paraplegia has declined in recent years, it is still a serious complication (3). Recently, the incidence of paraplegia was found to be higher in patients with a history of vascular surgical disease because of the lack of a collateral source of blood flow to the spinal cord, which has a large network (4). Thus, many factors are involved in the development of surgically induced paraplegia, and little is known about how to reduce the rate of this outcome (5). In this study, we analyzed the effectiveness of CSFD in preventing paraplegia. We retrospectively evaluated the neurological recovery of patients who underwent CSFD after thoracic aortic or thoracoabdominal aortic aneurysm surgery and determined which individuals developed symptoms of paraplegia. We also considered complications of CSFD, as well as short-term/long-term life expectancy. This study aimed the comparison between preoperative and postoperative CSFD. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-631/rc).
Methods
Study population
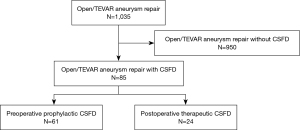
Data were collected from hospital admission medical records. All patients had been followed up as outpatients at our medical center. We reviewed data from a total of 1,035 patients who underwent surgery, including graft replacement (GR) and thoracic endovascular aortic repair (TEVAR) for thoracic aorta or thoracoabdominal aorta, between January 2006 and December 2022. Of the total study population, 85 patients underwent CSFD around the time of surgery (Figure 1). There were 61 preoperative CSFD patients and 24 postoperative CSFD patients. Sufficient data for analyses were available from 85 patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Committee on Ethics of Medicine of Sapporo Medical University (No. 352-14). Written informed consent was collected from all the subjects participating in the study.
Procedure for thoracic aortic aneurysm (TAA) and thoraco-abdominal aortic aneurysm (TAAA)
Patient selection for GR or TEVAR was dependent on a number of factors, such as age, comorbidities, the surgeon’s decision, device availability, and anatomical suitability. In this series, GR was performed in 61 patients (72%), and TEVAR was performed in 24 patients (28%). Cases were elective for 76 patients (89%) and emergent for nine patients (11%).
The evaluation of spinal cord insufficiency (SCI) and major morbidities
In all cases, the patients were evaluated by our team immediately after the identification of suspected SCI. The degree of SCI was classified according to the manual muscle test (MMT).
Prophylactic strategy for paraplegia/paraparesis
The AKA was identified preoperatively via contrast-enhanced computed tomography (CT), except in emergency cases. In all open thoracotomy procedures, the MEP was used to immediately detect spinal cord ischemia, regardless of whether the AKA was included in the scope of replacement. When the AKA was included in the replacement area, prophylactic preoperative CSFD was initiated on the day before surgery, and the AKA was selectively perfused during surgery to reconstruct the artificial vessel. CSFD was started immediately after anastomosis. The drainage was adjusted so that the cerebrospinal fluid (CSF) pressure did not exceed 12 cmH2O based on the height of the right atrium. When the drainage volume exceeded 30 mL/1 hour, the height was adjusted to raise the CSF pressure by 2 cmH2O. Conversely, when the drainage volume was low, the CSF pressure was lowered by 2 cmH2O and the minimum value was set at 6 cmH2O. The drainage tube was removed at 48 hours postoperatively, and CSFD was not conducted preoperatively in TEVAR patients, regardless of whether the AKA was included in the treatment area. Preoperative CSFD was not placed in the TEVAR group because of the minimally invasive nature of stent grafting and the lower incidence of paraplegia compared with artificial vessel replacement, according to previous reports. Postoperative CSFD was initiated at the onset of postoperative paraplegia, with a management protocol that was the same as that for artificial vessel replacement. In all patients, the systolic blood pressure was consistently kept above 140 mmHg, the hemoglobin (Hb) was kept above 10 mg/dL, and the cardiac index (CI) was kept above 2.5 L/min/body surface area (BSA).
Statistical analysis
Continuous variables were reported as the means ± standard deviation. The categorical variables in the tables were presented as raw numbers (percentages). The categorical variables were compared using the χ2 and Fisher’s exact tests. We used the Kaplan-Meier method to assess the intermediate and long-term factors associated with patient survival. All calculations were performed using JMP version 16 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics
In this study, a total of 1,035 patients underwent open or endovascular surgery (Table 1). Of these, 85 patients underwent CSFD. The patient preoperative characteristics and demographics are shown in Table 1. We performed a total of 85 consecutive CSFD procedures from 2015 to 2022. The mean patient age at the time of CSFD was 56.7±12.3 years. The majority of the patients were male (81%), and 82% of all of the patients had systemic hypertension. Smoking was recorded in 66% of the patients, and connective tissue disease was found in 8%, chronic kidney disease in 42%, and prior cardiovascular events in 61% of the patients. The logistic EuroSCORE was 8.4±6.4.
Table 1
| Variables | Overall (n=85) |
|---|---|
| Age (years) | 56.7±12.3 |
| Male | 69 [81] |
| Body surface area (m2) | 1.71±0.19 |
| Hypertension | 70 [82] |
| Dyslipidemia | 11 [13] |
| Diabetes mellitus | 11 [13] |
| Smoking | 56 [66] |
| Connective tissue disease | 7 [8] |
| Coronary artery disease | 13 [15] |
| Chronic obstructive pulmonary disease | 4 [5] |
| Peripheral artery disease | 8 [9] |
| Chronic kidney disease | 36 [42] |
| Prior cerebrovascular event | 10 [12] |
| Prior cardiovascular event | 52 [61] |
| Prior ascending aortic surgery | 19 [22] |
| Prior distal aortic repair | 2 [2] |
| Prior endovascular DTA repair | 2 [2] |
| Prior open/endovascular abdominal aortic repair | 13 [15] |
| Prior open/endovascular TAAA repair | 4 [5] |
| Prior aortic dissection episode | 34 [40] |
| Logistic EuroSCORE | 8.4±6.4 |
Categorical data are presented as number [%] and continuous data as mean ± standard deviation. DTA, descending thoracic aorta; TAAA, thoraco-abdominal aortic aneurysm.
Procedure characteristics
The procedure characteristics are shown in Table 2. In terms of indications for surgery and prior history, degenerative aneurysm was recorded in 50%, acute dissection in 5%, chronic dissection in 40%, and rupture in 5% of the patients. The surgery was elective in 89% of the patients, and urgent in 11% of the sample. An open repair surgery was conducted in 72% of the patients and an endovascular repair was performed in 28% of the patients. Range of aortic repair was shown in Table 2. The mean operation time for the open repair procedure was 551 min, while that for the endovascular repair was 159 min. The CSFD was preoperative in 72% and postoperative in 28% of the patients. For patients who underwent postoperative CSFD, the time from the end of the surgery to the detection of paraplegia/paraparesis was 9.8 hours.
Table 2
| Variables | Overall (n=85) |
|---|---|
| Indications for surgery and prior history | |
| Degenerative aneurysm | 43 [50] |
| Acute dissection | 4 [5] |
| Chronic dissection | 34 [40] |
| Rupture | 4 [5] |
| Urgency | |
| Elective | 76 [89] |
| Emergent | 9 [11] |
| Procedure | |
| Open repair | 61 [72] |
| Endovascular repair | 24 [28] |
| Range of aortic repair | |
| Ascending to total arch | 7 [8] |
| Descending aorta | 35 [41] |
| Crawford I | 4 [5] |
| Crawford II | 6 [7] |
| Crawford III | 20 [24] |
| Crawford IV | 13 [15] |
| Operation time of open repair (min) | 551±21 |
| CPB time of open repair (min) | 198±11 |
| Operation time of endovascular repair (min) | 159±35 |
| Timing of CSFD | |
| Preoperative CSFD | 61 [72] |
| Postoperative CSFD | 24 [28] |
| Time from end of surgery to detect paraplegia/paraparesis (hours) | 9.8±1.2 |
Categorical data are presented as number [%] and continuous data as mean ± standard deviation. CPB cardiopulmonary bypass; CSFD, cerebrospinal fluid drainage.
Postoperative outcomes
After the surgery, the rate of in-hospital death was 7%, that of transfer to the hospital was 19%, and discharge by foot was 74%. The mean number of days spent in the hospital was 25. At discharge, paraplegia was present in 8%, paraparesis in 13%, and major complications related to CSFD (cerebral bleeding or infection) in 2% of all patients.
Patients with paraplegia/paraparesis who were ineligible for CSFD
As shown in Figure 1, there were 950 patients who did not undergo CSFD. Among these patients, 20 were not eligible for CSFD, and developed paraplegia/paraparesis for various other reasons, including active bleeding after surgery, delayed awakening caused delayed judgment of paralysis, and cerebral stroke. In these 20 cases, 19 patients developed paraplegia and one patient developed paraparesis.
Effects of postoperative CSFD
In this sample, 24 patients underwent postoperative CSFD to treat paraplegia/paraparesis. Figure 2 shows box-and-whisker plots of the mean MMT scores of the postoperative CSFD group, measured before initiation of CSFD, just after CSFD, and at discharge. The mean MMT score before CSFD was 0.8, just after CSFD was 2.4, and at discharge was 3.0. Therefore, postoperative CSFD was associated with higher MMT scores compared with preoperative CSFD. We divided patients into two groups, no preoperative and postoperative CSFD and No preoperative CSFD and Postoperative CSFD done who were all diagnosed as paraplegia. The results are shown in Table 3. Of 27 patients who developed paraplegia, 13 remained paraplegia without CSFD. In contrast, of the 14 patients who underwent postoperative CFSD, 4 showed a slight recovery of MMT, but 10 remained paraplegia.
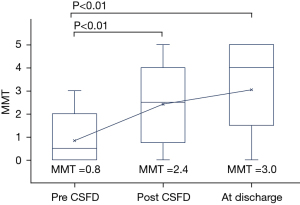
Table 3
| Variables | No preoperative and postoperative CSFD (n=13) | No preoperative CSFD and postoperative CSFD done (n=14) |
|---|---|---|
| Preoperative MMT | 5 | 5 |
| Just after operation MMT | 0 | 0 |
| At discharge MMT | 0 | 0.57 [0–2] |
Continuous data are shown as mean [range]. MMT, manual muscle test; CSFD, cerebrospinal fluid drainage.
Survival in patients who underwent postoperative CSFD
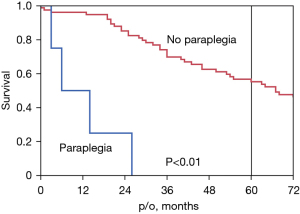
Figure 3 shows the survival curve for all-cause mortality in patients who underwent postoperative CSFD (n=24). We compared the paraplegia and no paraplegia groups using the Log-rank test, P<0.01. The 5-year survival rate was 0% in the paraplegia group and 55% in the no paraplegia group.
Survival in patients who underwent preoperative versus postoperative CSFD
Figure 4 shows the survival curve for all-cause mortality in patients who underwent preoperative CSFD (n=61) and postoperative CSFD (n=24). We compared the preoperative CSFD group with the postoperative CSFD group using the Log-rank test, P=0.57. The 5-year survival rate was 55% in the preoperative CSFD group versus 52% in the postoperative CSFD group. Postoperative outcomes were shown in Table 4.
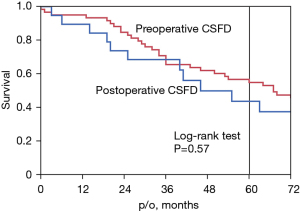
Table 4
| Variables | Overall (n=85) |
|---|---|
| In-hospital death | 6 [7] |
| Transfer to hospital | 16 [19] |
| Discharge by foot | 63 [74] |
| In-hospital days | 25 [18–42] |
| Paraplegia at discharge | 7 [8] |
| Paraparesis at discharge | 11 [13] |
| Major complications with CSFD (cerebral bleeding or infection) | 2 [2] |
Categorical data are presented as number [%] and continuous data as mean [range]. CSFD, cerebrospinal fluid drainage.
Discussion
SCI is a serious complication of open aortic surgery and TEVAR, and has been reported to occur in 20% of open thoracic aortic surgeries (6). Various strategies have been devised to reduce SCI, including CSFD, cooling, intercostal vessel reimplantation, and elevated mean arterial pressure (MAP) during open thoracic surgery. Many studies have suggested that there exists a relationship between CSFD during thoracic aortic repair and the development of SCI. The rationale for CSFD is that the spinal cord perfusion pressure is the difference between the MAP and CSF pressure (7).
COPS treatment was introduced for use during thoracoabdominal aortic aneurysm surgery, as reported by Estrera et al. Although CSFD is invasive, it is not difficult to achieve good oxygenation and high patient arterial pressure. If other treatments play a significant role in the prevention of SCI, it may be possible to avoid invasive CSFD (5).
The use of CSFD prophylactically during aortic laparotomy is controversial, and many studies have focused on this topic. For instance, Coselli et al. conducted a large randomized trial, and showed a significant reduction in SCI with CSFD (13% versus 2.6%, P=0.03) (8). The benefit of prophylactic CSFD in open aortic surgery has also been established via a meta-analysis (9). Indeed, many reports have examined the use of preoperative CSFD, with acceptable results. However, complications such as cerebral hemorrhage and infection have been reported, indicating that CSFD should be avoided unless necessary (10). If postoperative CSFD is as effective as preoperative CSFD, then postoperative CSFD should be prioritized. This would limit the use of preoperative CSFD to high-risk cases. To examine the plausibility of this suggestion, we examined the effectiveness of postoperative CSFD.
In this study, we examined data from 1,035 patients who underwent open thoracic replacement or stent grafting, 85 (8%) of whom had undergone CSFD implantation. Among the CSFD implantation patients, 61 (72%) underwent preoperative CSFD and 24 (28%) underwent postoperative CSFD. Patient background data are shown in Table 1. The prevalence of connective tissue disease and chronic kidney disease was 8% and 40%, respectively. Characteristically, 61% of the patients had experienced a previous cardiovascular event.
Indications for surgery and prior history included 43 cases of degenerative aneurysm (50%) and 34 cases of chronic dissection (40%). Most of the cases in this series (76 cases, 89%) underwent elective surgery.
In 24 cases of postoperative CSFD, the MMT score was significantly improved, as shown in Table 2. Of the 24 postoperative CSFD patients, 13 underwent open surgery and 11 underwent TEVAR. However, six patients remained paraplegic postoperatively after CSFD. Five of the six patients underwent open surgery, and one patient underwent TEVAR. In other words, 5 of 13 open cases (38%) who developed paraplegic symptoms remained paraplegic postoperatively, while only 1 of 11 TEVAR cases (9%) remained paraplegic (P=0.10). All six patients who underwent open surgery had the AKA in the treatment range and had undergone previous abdominal aortic vascular surgery. There were no major complications related to postoperative CSFD. In this series, there were 20 cases in which CSFD was not performed because of delayed postoperative arousal or excessive postoperative bleeding, although CSFD was originally considered necessary because of paraplegia. Of these patients, 19 developed paraplegia, and the remaining patient had incomplete paraplegia. In contrast, only one patient (2%) in the preoperative CSFD group developed paraplegia, and major complications were a cerebral hemorrhage case and a sepsis case (11).
Figure 3 shows the survival curves for patients with and without paraplegia. The 5-year survival rate in the patients with paraplegia was 0%, which reaffirms the poor prognosis of this patient group (8). Figure 4 shows the survival curves for the patients who underwent preoperative versus postoperative CSFD. There was no difference in survival between the two groups, although there was a difference in the incidence of paraplegia. In other words, postoperative CSFD improved MMT scores and avoided paraplegia in both the open and TEVAR groups, with no significant difference. Furthermore, there was no significant difference in long-term survival between the preoperative and postoperative CSFD groups, indicating that similar long-term outcomes can be expected between the groups. The purpose of this study was to demonstrate the effectiveness of postoperative CSFD in patients with paraplegia, as shown in Table 3. As shown in Figure 4, the survival rate of patients treated with postoperative CSFD was unchanged compared with that of patients treated with preoperative CSFD, suggesting that CSFD is safe.
By confirming the effectiveness of postoperative CSFD, we thought it would be possible to examine its effectiveness in comparison with preoperative CSFD cases. We examined the efficacy of 61 CSFD cases introduced preoperatively in this study and found only one case of paraplegia. In contrast, 6 of 24 postoperative CSFD patients (25%) remained paraplegic, as described above. In light of the above, postoperative CSFD may be considered ineffective, but it may have improved the 75% of patients who would likely have become paraplegic without the introduction of CSFD. The MMT outcomes of the 24 patients who underwent postoperative CSFD were followed closely and are considered scientifically credible.
In the future, we hope to compare data from patients who underwent preoperative versus postoperative CSFD, after matching for other variables via a randomized controlled trial (RCT) or propensity score matching (PSM). Patients who undergo other arterial surgeries before surgery are at high risk of paraplegia, and we hope to find a treatment other than CSFD or COPS to avoid paraplegia in this patient group.
Limitations
This was a single-center, retrospective study, and although all of the patients underwent CSFD implantation, the background of the patients was heterogeneous because of differences in the surgical procedures performed. However, the neurological evaluations of patients following CSFD implantation were conducted in a consistent manner. Because this was a retrospective study, it is not possible to determine whether preoperative CSFD is superior to postoperative CSFD after the onset of paraplegia symptoms. In the future, we hope to compare data from patients who underwent preoperative versus postoperative CSFD, after matching for other variables via a RCT or PSM. Patients who undergo other arterial surgeries before surgery are at high risk of paraplegia, and we hope to find a treatment other than CSFD or COPS to avoid paraplegia in this patient group.
Conclusions
We investigated the efficacy of postoperative CSFD in patients with paraplegia after thoracic and thoracoabdominal aortic aneurysm surgery at our hospital. To the best of our knowledge, this is the first study to examine the effect of postoperative CSFD on neurological prognosis. Postoperative CSFD contributed to the recovery of MMT score. CSFD should be inserted for postoperative SCI if the indicated conditions were met.
Acknowledgments
We thank Sydney Koke, MFA, from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-631/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-631/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-631/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-631/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Committee on Ethics of Medicine of Sapporo Medical University (No. 352-14). Written informed consent was collected from all the subjects participating in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Estrera AL, Miller CC 3rd, Huynh TT, et al. Preoperative and operative predictors of delayed neurologic deficit following repair of thoracoabdominal aortic aneurysm. J Thorac Cardiovasc Surg 2003;126:1288-94. [Crossref] [PubMed]
- Aucoin VJ, Bolaji B, Novak Z, et al. Trends in the use of cerebrospinal drains and outcomes related to spinal cord ischemia after thoracic endovascular aortic repair and complex endovascular aortic repair in the Vascular Quality Initiative database. J Vasc Surg 2021;74:1067-78. [Crossref] [PubMed]
- Abdelbaky M, Papanikolaou D, Zafar MA, et al. Safety of perioperative cerebrospinal fluid drain as a protective strategy during descending and thoracoabdominal open aortic repair. JTCVS Tech 2021;6:1-8. [Crossref] [PubMed]
- Kawaharada N, Ito T, Koyanagi T, et al. Spinal cord protection with selective spinal perfusion during descending thoracic and thoracoabdominal aortic surgery. Interact Cardiovasc Thorac Surg 2010;10:986-91. [Crossref] [PubMed]
- Estrera AL, Sheinbaum R, Miller CC 3rd, et al. Neuromonitor-guided repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2010;140:S131-5; discussion S142-6. [Crossref] [PubMed]
- Wong CS, Healy D, Canning C, et al. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg 2012;56:1438-47. [Crossref] [PubMed]
- Acher C, Acher CW, Marks E, et al. Intraoperative neuroprotective interventions prevent spinal cord ischemia and injury in thoracic endovascular aortic repair. J Vasc Surg 2016;63:1458-65. [Crossref] [PubMed]
- Coselli JS, Green SY, Price MD, et al. Spinal cord deficit after 1114 extent II open thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2020;159:1-13. [Crossref] [PubMed]
- Cinà CS, Abouzahr L, Arena GO, et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aortic aneurysm surgery: a systematic review and meta-analysis. J Vasc Surg 2004;40:36-44. [Crossref] [PubMed]
- Kärkkäinen JM, Cirillo-Penn NC, Sen I, et al. Cerebrospinal fluid drainage complications during first stage and completion fenestrated-branched endovascular aortic repair. J Vasc Surg 2020;71:1109-18.e2. [Crossref] [PubMed]
- Song S, Song SW, Kim TH, et al. Effects of preemptive cerebrospinal fluid drainage on spinal cord protection during thoracic endovascular aortic repair. J Thorac Dis 2017;9:2404-12. [Crossref] [PubMed]


