
Tumor-to-thoracic height ratio as an easy method to predict the feasibility of reduced-port video-assisted thoracic surgery for mediastinal lesions in children: a single-center experience
Introduction
Video-assisted thoracic surgery (VATS) has become a major approach for both adults and children (1,2). The important advantages of VATS over open thoracotomy in children include fewer musculoskeletal sequelae and better cosmetic outcomes (3,4), long-term pulmonary function (4), and lesser use of epidural anesthesia (5). Typically, it is performed using three or four access ports (6). In the last few decades, reduced-port VATS (RP-VATS), including uniportal VATS (7,8), two-port VATS, and needlescopic VATS [such as the one-window and puncture method (1WPM)] (9,10), have been developed to minimize surgical invasiveness. The theoretical advantages of RP-VATS are minimal surgical invasiveness (because the wound does not require suturing), minimal scarring, and minimized pain (9,11-13). Nevertheless, VATS can occasionally be difficult in children because the lesion occupies a small thoracic cavity, thereby limiting the working space (10). As a result, uniportal VATS is yet to be adopted for children (14), except for diagnostic procedures (15).
Mediastinal tumors are rare and often difficult to resect in children due to variability in the tumor location as well as the anatomical limitations of the mediastinum. For embryonal tumors, including neuroblastomas, image-defined risk factors and a larger tumor volume (TV) have been reported to discourage minimally invasive surgery (16-18). However, no imaging criteria are currently available to help select the optimal surgical approach for other pediatric mediastinal lesions.
Occupancy of the thoracic cavity by the tumor, which leads to surgical difficulty, depends not only on the tumor itself, but also on the size of the thoracic cavity. Therefore, we hypothesized that the tumor-to-thoracic height ratio (TTH ratio) better represents the occupancy of the thoracic cavity by the tumor and may allow easier prediction of the feasibility of RP-VATS in children. Accordingly, the aim of this study was to assess the feasibility of RP-VATS for the resection of mediastinal lesions in children in association with the TTH ratio. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-515/rc).
Methods
Study design and patients
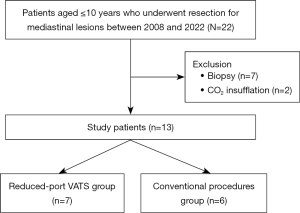
This study was approved by the Institutional Review Board of Hokkaido University Hospital (approval No. 022-0078) and was performed in accordance with the Declaration of Helsinki (as revised in 2013). The requirement of obtaining individual informed consent was waived due to the study’s retrospective nature; however, information about this study was made readily available to the legal guardians of the patients on the institutional website. Furthermore, the opportunity to opt out was guaranteed; patients who opted out were excluded from the study. All patients aged ≤10 years who underwent resection of mediastinal lesions at our institute between January 2008 and August 2022 were retrospectively reviewed. Seven patients who underwent diagnostic procedures were excluded from this study. Furthermore, two more patients who underwent VATS with carbon dioxide (CO2) insufflation were also excluded. This is because the procedure requires airtight access ports, which were of a minimum size of 5 mm at our institute; thus, CO2 insufflation was deemed incompatible with RP-VATS (which involves 3 mm access ports). Ultimately, thirteen patients were included in this study (Figure 1).
Surgical procedures
Surgery was performed under general anesthesia in the lateral decubitus position. When possible, one-lung ventilation was applied with either a double-lumen tube, a bronchial blocker, or main bronchial intubation. The patients were divided into two groups, including a RP-VATS group and a conventional procedures group. RP-VATS included uniportal VATS and the 1WPM, while conventional procedures included multi-portal VATS and open thoracotomy.
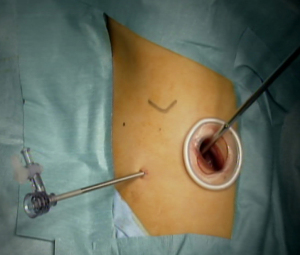
In the RP-VATS group, an initial 5–40 mm incision was made on the anterior or posterior midaxillary line of the fifth or sixth intercostal space. An Alexis wound retractor XS™ (Applied Medical, Rancho Santa Margarita, CA, USA) or a LAPPROTECTOR™ mini-mini (Hakko Medical, Tokyo, Japan) was used to protect the wound. A 30° rigid scope (diameter: 3 or 5 mm; Olympus Medical Science, Tokyo, Japan) was used for intrathoracic observation to confirm the indications for RP-VATS. If the surgery was completed with the initial incision, it was defined as a uniportal VATS. Additional 3-mm punctures were made for the needle scope and other instruments if insertion through the initial incision site was insufficient, which was defined as 1WPM (Figure 2). Conventional multiportal VATS was defined when additional incisions over 3 mm were made in addition to the initial incision.
We generally considered RP-VATS first whenever possible, regardless of the TTH ratio; however, we opted for conventional VATS or open thoracotomy when the tumor seemed to infiltrate the surrounding structures or encased great vessels. When RP-VATS was deemed difficult to perform, we converted to conventional multi-portal VATS or open thoracotomy.
Data collection and definitions
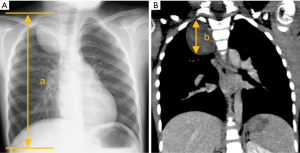
Data were collected from the medical records of Hokkaido University Hospital. Thoracic height was calculated using chest radiography because it was difficult to measure the maximum diameter of the thoracic height using the coronal section of a chest computed tomography (CT), defined as the diameter from the apex of the lung to the bottom of the costophrenic angle (Figure 3A). The tumor height was calculated using the coronal section of the chest CT (Figure 3B) because the tumor height was difficult to measure using chest radiography in some cases due to the ambiguous margin of the lesion. The TTH ratio was calculated as tumor height divided by thoracic height (TTH ratio = b/a × 100%) (Figure 3). TV was calculated using the three dimensions from cross-sectional CT imaging (TV = length × width × height × π/6 mL), as previously reported (17). Postoperative complications were assessed using the Clavien-Dindo classification (19). Furthermore, the mediastinal compartments were divided into prevascular (anterior), visceral (middle), and paravertebral (posterior) compartments, which were defined by the International Thymic Malignancy Interest Group (20).
Statistical analysis
Statistical analyses were performed using R version 4.1.3 (R Foundation for Statistical Computing Vienna, Austria). Categorical data are presented as numbers and proportions, and continuous data are presented as medians and interquartile ranges (IQR). The Mann-Whitney U test was used to compare the two groups, and frequencies were compared using the Fisher’s exact test for categorical variables. A logistic regression model was used in the univariate and multivariate analyses to evaluate the significance of preoperative variables as independent prognostic factors for the feasibility of reduced-port VATS. Considering the low number of patients included in this study, only age (in months) and the TTH ratio were included in the multivariate analysis. All analyses were two-tailed, and statistical significance was set at P<0.05.
Results
Patient characteristics
A total of seven patients in the RP-VATS group (two uniportal VATS and five 1WPM) and six in the conventional procedures group (three conventional multiportal VATS and three open thoracotomy) were included. The baseline characteristics of the patients are summarized in Table 1. The median age in months was 80 months (IQR, 47.0–94.0) months for the RP-VATS group and 67 months (IQR, 23.8–89.3) months for the conventional procedures group, which was not significantly different. Three males (42.9%) in the RP-VATS group and one male (16.7%) in the conventional procedures group were included. Body height [median (IQR): 114.0 (93.4–127.7) vs. 106.3 (79.5–116.9) cm, respectively], and body weight [median (IQR): 17.4 (13.8–24.0) vs. 17.2 (10.8–24.6) kg, respectively] were not significantly different between the two groups. No patients had obvious chromosomal abnormalities or congenital lung disease. The RP-VATS group included three bronchogenic cysts, three ganglioneuroblastomas, and a ganglioneuroma, whereas the conventional procedures group included a cystic lymphangioma, a rhabdoid tumor, a ganglioneuroma, and three ganglioneuroblastomas. In both groups, the most frequent tumor location was the posterior mediastinum, whereas no anterior mediastinal tumors were included. Although TV was not significantly different between the two groups, the TTH ratio was significantly lower in the RP-VATS group than in the conventional procedures group [median (IQR): 26.3 (24.5–34.5) vs. 50.8 (47.0–53.1), respectively; P=0.007].
Table 1
| Variables | Reduced-port (n=7) | Conventional (n=6) | P value |
|---|---|---|---|
| Age, months | 80.0 (47.0, 94.0) | 67.0 (23.8, 89.3) | 0.78† |
| Sex | 0.56‡ | ||
| Male | 3 (42.9) | 1 (16.7) | |
| Female | 4 (57.1) | 5 (83.3) | |
| Body height, cm | 114.0 (93.4, 127.7) | 106.3 (79.5, 116.9) | 0.57† |
| Body weight, kg | 17.4 (13.8, 24.0) | 17.2 (10.8, 24.6) | 0.67‡ |
| Pathological findings | 0.26‡ | ||
| Bronchogenic cyst | 3 (42.9) | 0 (0.0) | |
| Cystic lymphangioma | 0 (0.0) | 1 (16.7) | |
| Rhabdoid tumor | 0 (0.0) | 1 (16.7) | |
| Ganglioneuroma | 1 (14.3) | 1 (16.7) | |
| Ganglioneuroblastoma | 3 (42.9) | 3 (50.0) | |
| Tumor location (anterior, middle, posterior) | 0, 2, 5 | 0, 1, 5 | 1.0‡ |
| Thoracic height, mm | 179.0 (152.0, 203.0) | 151.0 (110.5, 175.0) | 0.32† |
| Maximum tumor diameter, mm | 47.0 (41.0, 52.0) | 77.0 (61.8, 93.0) | 0.045† |
| Tumor volume, mL | 28.8 (18.4, 38.6) | 112.9 (22.9, 189.0) | 0.39† |
| Tumor-to-thoracic height ratio | 26.3 (24.5, 34.5) | 50.8 (47.0, 53.1) | 0.007† |
Data are presented as number, number (%) or median (interquartile range). †, continuous variables were compared using the Mann-Whitney U test. ‡, frequencies were compared using the Fisher’s exact test for categorical variables.
Perioperative outcomes
The perioperative outcomes are summarized in Table 2. No patients underwent conversion from RP-VATS to conventional VATS. In one patient who was initially started on uniportal VATS with a 40 mm incision, the incision had to be enlarged to 45 mm for specimen extraction. One patient underwent conversion from RP-VATS to thoracotomy due to a large tumor size; furthermore, one of the two patients who were initially started on conventional VATS also underwent conversion to thoracotomy. The operating time [median (IQR): 110 (93.5–142.5) vs. 287.5 (244.8–326.5) min, respectively; P=0.01] and duration of drainage [median (IQR): 0.0 (0.0–0.5) vs. 2.0 (2.0–2.8) days, respectively; P=0.003] were significantly shorter and blood loss [median (IQR): 0.0 (0.0–0.0) vs. 55 (35.0–596.3) mL, respectively; P=0.001] was significantly lower in the RP-VATS group than in the conventional procedures group. Although the incidence rate of complications over grade 2 was not different between the two groups, two complications (28.6%), including a Horner syndrome and a pyothorax, occurred in the RP-VATS group. The postoperative observation period was also not significantly different between the two groups [median (IQR): 93.0 (46.5–1,822.0) vs. 1,119.0 (888.8–1,527.8) days, respectively; P=0.39]. No patients in the RP-VATS group experienced recurrence, whereas one patient in the conventional procedures group experienced recurrence during the observation period.
Table 2
| Variables | Reduced-port (n=7) | Conventional (n=6) | P value |
|---|---|---|---|
| Initial procedure | |||
| VATS | 7 (100.0) | 3 (50.0) | |
| Conversion to thoracotomy | 1 | 1 | |
| Open thoracotomy | 0 (0.0) | 3 (50.0) | |
| One-lung ventilation | 0.56b | ||
| Yes | 6 (85.7) | 4 (66.7) | |
| No | 1 (14.3) | 2 (33.3) | |
| Operating time, min | 110.0 (93.5, 142.5) | 287.5 (244.8, 326.5) | 0.01a |
| Blood loss, mL | 0.0 (0.0, 0.0) | 55.0 (35.0, 596.3) | 0.001a |
| Duration of drainage, days | 0.0 (0.0, 0.5) | 2.0 (2.0, 2.8) | 0.003a |
| Length of hospital stay, days | 4.0 (3.0, 5.5) | 8.50 (5.50, 10.75) | 0.13a |
| Observation period, days | 93.0 (46.5, 1,822.0) | 1,119.0 (888.8, 1,527.8) | 0.39a |
| Complications of grade >2 | 1.0b | ||
| Yes | 2 (28.6) | 2 (33.3) | |
| Horner syndrome | 1 | 0 | |
| Pyothorax | 1 | 0 | |
| Chylothorax | 0 | 1 | |
| Atelectasis | 0 | 1 | |
| No | 5 (71.4) | 4 (66.7) | |
| Recurrence | 0.46b | ||
| Yes | 0 (0.0) | 1 (16.7) | |
| No | 7 (100.0) | 5 (83.3) |
Data are presented as number, number (%) or median (interquartile range). a, continuous variables were compared using the Mann–Whitney U-test. b, frequencies were compared using the Fisher’s exact test for categorical variables. VATS, video-assisted thoracic surgery.
Predicting factors of RP-VATS completion
In the univariate analysis using a logistic regression model, a lower TTH ratio was significantly associated with the completion of RP-VATS [odds ratio: 0.775; 95% confidence interval (CI): 0.528–0.926; P=0.049], whereas age, height, body weight, availability of one-lung ventilation, maximum tumor diameter, and TV were not (Table 3). Moreover, after adjusting for age, a lower TTH ratio remained significantly associated with the completion of RP-VATS (odds ratio: 0.776, 95% CI: 0.529–0.926, P=0.048; Table 3).
Table 3
| Variables | Crude | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age, months | 1.005 | 0.976–1.037 | 0.72 | 0.996 | 0.941–1.049 | 0.88 | |
| Body height, mm | 1.012 | 0.968–1.062 | 0.60 | ||||
| Body weight, kg | 1.021 | 0.878–1.195 | 0.78 | ||||
| One-lung ventilation (yes/no) | 3.000 | 0.214–78.77 | 0.43 | ||||
| Maximum tumor diameter, mm | 0.928 | 0.831–0.991 | 0.07 | ||||
| Tumor volume, mL | 0.980 | 0.935–0.999 | 0.14 | ||||
| Tumor-to-thoracic height ratio | 0.775 | 0.528–0.926 | 0.049 | 0.776 | 0.529–0.926 | 0.048 | |
OR, odds ratio; CI, confidence interval.
Discussion
Theoretically, the benefits of VATS are greater with RP-VATS. However, uniportal VATS is rarely performed in children (14). This is partly due to the smaller dimensions of their body (which afford a smaller working space) as well as varieties in the size, location, and tumor anatomy of pediatric mediastinal lesions (10,21). Uniportal VATS requires ingenuity in securing the field of view because the camera and forceps are inserted from one direction (10). Additionally, the narrow intercostal space in children limits the movement of the instruments inserted through a single incision (9,10). In this regard, the additional 3 mm ports in 1WPM can serve as a camera for confirmation of the surgical field, and needle forceps can also be inserted. Compared with patients undergoing conventional VATS, patients undergoing needlescopic VATS reportedly experience lesser postoperative pain (11) and residual neuralgia (13). The surgical invasiveness with a 3 mm port is minor because the wound does not require suturing, produces minimal scarring, and causes minimal pain (9,11,12). Therefore, 1WPM alleviates the limitations of uniportal VATS and expands the indications for RP-VATS in children with minimal invasiveness.
The drawbacks of the 3 mm thoracoscope are its narrow field of view and poor image brightness when used in adults (9,22). However, in small children, it provides sufficient brightness and magnification because the thoracic cavity in small children is much smaller than that in adults.
Even if a surgical procedure is successfully completed with three or four small access ports, enlarging the incision is sometimes necessary to extract the tumor without damaging it. Therefore, we made an initial 5–40 mm incision for uniportal VATS or 1WPM. This initial incision allowed multiple instruments to be inserted through a single wound. However, this method makes CO2 insufflation difficult; therefore, patients who underwent CO2 insufflation were excluded from the study.
In the present study, the TTH ratio was associated with the completion of RP-VATS in the resection of mediastinal lesions in children, although other factors, including patient age, height, body weight, availability of one-lung ventilation, tumor diameter, and TV, were not. Although TV may also be an important factor in predicting complications (16-18), patient age, body size, and availability of one-lung ventilation may also affect the difficulty of the surgical procedure. This study suggests that the TTH ratio is a simpler and more sensitive index for predicting the feasibility of RP-VATS because it better represents thoracic occupancy by the lesion. Only one patient who was initially started on RP-VATS required conversion to thoracotomy due to a large tumor; another patient who initially underwent conventional VATS also required conversion to open thoracotomy. This suggests that mediastinal lesions with larger TTH ratios are difficult to resect using VATS, even if the sizes of the access ports are large. In this study, chest radiography and chest CT were used to calculate the thoracic height and tumor height accurately; however, calculating both with radiography alone may be an easier method for predicting the feasibility of RP-VATS. The optimal modality for these calculations should be investigated further. In addition, the maximum lesion diameter, instead of the lesion height, may be used for calculating the TTH ratio. In this study, we chose to use the lesion height because it is simpler to calculate and can be estimated using chest radiography.
Compared with the conventional procedures group, the RP-VATS group in this study experienced a shorter operating time, lower blood loss, and shorter duration of chest drainage, with similar complication rates. These findings may be attributed to both the surgical technique used and the patients’ background characteristics. RP-VATS may reduce the operation time because the time required to open and close the wound is generally less than that required in open thoracotomy. Furthermore, the blood loss in RP-VATS may be lesser because the muscle incision made is minimal. Conversely, surgery is generally less difficult in patients with a low TTH ratio; therefore, the operating time may be shorter and blood loss may be lesser. Additionally, no recurrence occurred in the RP-VATS group during the observation period, whereas a patient with nodular ganglioneuroblastoma in the conventional procedures group developed a local recurrence. The RP-VATS and conventional procedures groups differed in terms of the access port and TTH ratio but not in terms of the complication rate. These findings suggest that RP-VATS can be performed safely and with minimal invasiveness in appropriately selected patients. Therefore, RP-VATS may be feasible for mediastinal lesions with a low TTH ratio and no apparent invasion into the surrounding structures. RP-VATS can be considered whenever possible; however, in difficult cases, conventional VATS or open thoracotomy should always be considered instead.
Recently, robot-assisted thoracic surgery (RATS) has been performed for pediatric mediastinal lesions (23,24). However, a major limitation of RATS for infants and small children is the lack of dedicated instruments (23). Although only few studies on RATS for pediatric mediastinal lesions have been reported, RATS will likely become more common with advances in technology. Future studies are required to compare the outcomes of thoracotomy, conventional VATS, RP-VATS, and RATS.
Our study had several limitations. First, this was a single-center retrospective study with a small sample size. Other factors, such as the location, histological characteristics, invasion status of the tumor; availability of one-lung ventilation; and surgeon’s experience may further affect the feasibility of RP-VATS. Although the baseline characteristics between the two groups were not significantly different, the patients’ characteristics were varied; these differences may have affected the study findings due to its small sample size. Additionally, patients were allocated to the groups at the surgeon’s discretion. Therefore, whether a low TTH ratio can be an effective predictor of the feasibility of RP-VATS should be investigated further. Currently, our findings only suggest a low TTH ratio to be a predictor of the feasibility of RP-VATS; this must be confirmed in prospective multicenter studies with larger sample sizes. Therefore, the feasibility should be well determined preoperatively. In addition, we could not calculate the optimal cutoff TTH ratio due to the small sample size. Second, anterior mediastinal lesions were not included in this study. Although this study indicates the feasibility of RP-VATS for middle and posterior mediastinal lesions with a lower TTH ratio, its feasibility for anterior mediastinal lesions should be investigated further. Third, the postoperative follow-up period in this study varied according to the patients’ diseases; furthermore, some patients travelled from another city and requested postoperative follow-up to be conducted at another hospital. This could have further led to the differences in the postoperative follow-up period in this study. Therefore, further studies are necessary to determine the recurrence rate over a long-term follow-up. Finally, we excluded patients who underwent CO2 insufflation; it may be beneficial if CO2 insufflation can be adapted to RP-VATS.
Conclusions
RP-VATS can be performed appropriately in carefully selected pediatric patients with middle and posterior mediastinal lesions. The TTH ratio may be a simple predictor of predicting the feasibility of RP-VATS, as it represents the occupancy of the thoracic cavity by the lesion. However, further studies are warranted to determine the criteria for the indications of RP-VATS in children, so that more children can benefit from the procedure.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-515/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-515/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-515/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lacher M, Kuebler JF, Dingemann J, et al. Minimal invasive surgery in the newborn: current status and evidence. Semin Pediatr Surg 2014;23:249-56. [Crossref] [PubMed]
- Rothenberg SS, Middlesworth W, Kadennhe-Chiweshe A, et al. Two decades of experience with thoracoscopic lobectomy in infants and children: standardizing techniques for advanced thoracoscopic surgery. J Laparoendosc Adv Surg Tech A 2015;25:423-8. [Crossref] [PubMed]
- Lawal TA, Gosemann JH, Kuebler JF, et al. Thoracoscopy versus thoracotomy improves midterm musculoskeletal status and cosmesis in infants and children. Ann Thorac Surg 2009;87:224-8. [Crossref] [PubMed]
- Lau CT, Wong KKY. Long-term pulmonary function after lobectomy for congenital pulmonary airway malformation: is thoracoscopic approach really better than open? J Pediatr Surg 2018;53:2383-5. [Crossref] [PubMed]
- Xie J, Wu Y, Wu C. Is thoracoscopy superior to thoracotomy in the treatment of congenital lung malformations? An updated meta-analysis. Ther Adv Respir Dis 2020;14:1753466620980267. [Crossref] [PubMed]
- Sihoe ADL. Video-assisted thoracoscopic surgery as the gold standard for lung cancer surgery. Respirology 2020;25:49-60. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [Crossref] [PubMed]
- Shiiya H, Ujiie H, Kato T, et al. Elective Uniportal Video-Assisted Thoracoscopic Lobectomy for Congenital Cystic Lung Disease in a 2-Year-Old Child Using One-Lung Ventilation. Indian J Surg 2022; [Crossref]
- Kaga K, Hida Y, Nakada-Kubota R, et al. Reduced port video-assisted thoracoscopic surgery using a needle scope for lung and mediastinal lesions. Interact Cardiovasc Thorac Surg 2013;17:268-72. [Crossref] [PubMed]
- Aragaki M, Kaga K, Hida Y, et al. Feasibility and safety of reduced-port video-assisted thoracoscopic surgery using a needle scope for pulmonary lobectomy- retrospective study. Ann Med Surg (Lond) 2019;45:70-4. [Crossref] [PubMed]
- Chou SH, Chuang IC, Huang MF, et al. Comparison of needlescopic and conventional video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2012;21:168-72. [Crossref] [PubMed]
- Ikeda Y, Miyoshi S, Seki N, et al. Needlescopic operation for partial lung resection. Ann Thorac Surg 2003;75:599-601. [Crossref] [PubMed]
- Chen JS, Hsu HH, Kuo SW, et al. Needlescopic versus conventional video-assisted thoracic surgery for primary spontaneous pneumothorax: a comparative study. Ann Thorac Surg 2003;75:1080-5. [Crossref] [PubMed]
- Ugolini S, Coletta R, Lo Piccolo R, et al. Uniportal Video-Assisted Thoracic Surgery in a Pediatric Hospital: Early Results and Review of the Literature. J Laparoendosc Adv Surg Tech A 2022;32:713-20. [Crossref] [PubMed]
- Fernandez-Pineda I, Seims AD, VanHouwelingen L, et al. Modified Uniportal Video-Assisted Thoracic Surgery Versus Three-Port Approach for Lung Nodule Biopsy in Pediatric Cancer Patients. J Laparoendosc Adv Surg Tech A 2019;29:409-14. [Crossref] [PubMed]
- Phelps HM, Ayers GD, Ndolo JM, et al. Maintaining oncologic integrity with minimally invasive resection of pediatric embryonal tumors. Surgery 2018;164:333-43. [Crossref] [PubMed]
- Gabra HO, Irtan S, Cross K, et al. Minimally invasive surgery for neuroblastic tumours: A SIOPEN multicentre study: Proposal for guidelines. Eur J Surg Oncol 2022;48:283-91. [Crossref] [PubMed]
- Liu T, Lv Z, Xu W, et al. Role of image-defined risk factors in predicting surgical complications of localized neuroblastoma. Pediatr Surg Int 2020;36:1167-72. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Carter BW, Tomiyama N, Bhora FY, et al. A modern definition of mediastinal compartments. J Thorac Oncol 2014;9:S97-101. [Crossref] [PubMed]
- Young S, Rettig RL, Hutchinson IV, et al. Surgical approach to pediatric mediastinal masses based on imaging characteristics. Pediatr Surg Int 2022;38:1297-302. [Crossref] [PubMed]
- Tagaya N, Kasama K, Suzuki N, et al. Video-assisted bullectomy using needlescopic instruments for spontaneous pneumothorax. Surg Endosc 2003;17:1486-7. [Crossref] [PubMed]
- Ballouhey Q, Villemagne T, Cros J, et al. Assessment of paediatric thoracic robotic surgery. Interact Cardiovasc Thorac Surg 2015;20:300-3. [Crossref] [PubMed]
- Svetanoff WJ, Bergus KC, Xia J, et al. Robotic-assisted resection of mediastinal tumors in pediatric patients. Semin Pediatr Surg 2023;32:151262. [Crossref] [PubMed]


