
Yiqi Yangjing recipe stimulates apoptosis while suppressing the energy metabolism via under-expression of PFKFB3 in A549 cells
Highlight box
Key findings
• This study demonstrated that Yiqi Yangjing recipe (YYR) promoted lung cancer cell apoptosis and inhibited energy metabolism by targeting PFKFB3.
What is known and what is new?
• The Warburg effect indicates that tumors obtain more energy for metabolism by activating glycolytic pathways. PFKFB3 is a key rate-limiting enzyme in glycolysis.
• Our preliminary experiments revealed that the good cytotoxic activity of YYR against the proliferation of A549 cells was related to PFKFB3. YYR was shown to be able to induce apoptosis of A549 cells, reduce extracellular acidification rate (ECAR), and inhibit cell metabolism. Additionally, YYR was shown to have a regulatory effect on mRNA and protein expressions of PFKFB3.
What is the implication, and what should change now?
• We need to embark on a more in-depth pharmacological study of YYR to determine the complex compound ingredients. YYR should be promoted in clinical practice to achieve efficacy in prolonging recurrent metastasis of lung cancer.
Introduction
Lung cancer is the most common malignancy worldwide in 2020 and the leading cause of cancer-related death (1), with non-small cell lung cancer (NSCLC) accounting for 80–90% of these (2). The incidence and mortality of lung cancer have been rising every year, consistently posing a threat to human health. Currently, the 5-year survival rate for lung cancer is only 20% (3,4). Great progress has been made in the diagnosis and treatment of lung cancer. Although surgical resection, radiotherapy, and chemotherapy are often used as the main treatments in clinical practice, the limitations (such as tolerance) and adverse reactions of these treatment methods cannot be ignored (5-8). In the clinical setting, combination regimens such as tyrosine kinase inhibitors (TKIs) and chemotherapy or immune checkpoint inhibitors (ICIs) have improved the progression-free survival (PFS) or overall survival (OS) compared with monotherapy (9,10). Therefore, it is of great significance for the management of lung cancer to find new alternatives or complementary drugs with good efficacy and fewer adverse reactions.
The etiology of lung cancer is not fully understood, but data have been suggesting that the occurrence of lung cancer is closely related to the disorder of cancer-related genes, mutations in DNA repair genes, growth factor signaling pathways, abnormal cell cycle regulation, and defects in apoptosis (11-13). One of the basic characteristics of the tumor microenvironment is hypoxia, as tumor cells usually rely on the glycolysis pathway for energy (14). PFKFB3, also known as the “regulator” of the body’s metabolism, is a key rate-limiting enzyme in glycolysis. A study has reported that blocking PFKFB3 significantly promotes the apoptosis of tumor cells (15). Therefore, the regulation of PFKFB3 gene expression could be researched as one alternative pathway toward the treatment of malignant tumors.
Yiqi Yangjing recipe (YYR) is a commonly used prescription for treating lung cancer in traditional Chinese medicine (TCM). A clinical study has found that YYR has an inhibitory effect on the proliferation, invasion, and metastasis of lung cancer cells in patients (16). Proteomic tests were performed on serum of patients in the YYR group before and after taking the medication. Three patients in the group were selected to be divided into group T1 before taking YYR and group T2 after taking YYR, and their serum proteomic tests were performed. We found that the changes in serum protein were mainly as follows: (I) Gene Ontology (GO)-enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) related pathways are closely related to glucose metabolism, apoptotic autophagy and pyruvate metabolism. (II) The biological processes, cell components and biological functions in KEGG related pathways showed that the lactate dehydrogenase pathway and glucose metabolic pathway before and after taking YYR could be further studied. YYR has been shown to decrease the expression levels of VEGF and HIF-1α in A549 cells (17), and effectively reduce the formation of tumor blood vessels and lymphatic vessels by inhibiting the Apelin/APJ signaling pathway in lung cancer cells (18). Although a few studies have focused on the effect of YYR on the expression of PFKFB3 in lung cancer cells, it is still not clear whether YYR can affect the apoptosis and glycolysis. As part of our continuing work toward the discovery of natural products against lung cancers via targeting PFKFB3, in our preliminary experiments we found that the good cytotoxic activity of YYR against the proliferation of A549 cells was related to PFKFB3. Therefore, this study aimed to clarify the anti-tumor effect and mechanism of YYR and to provide new insights for the prevention and treatment of lung cancer. We present this article in accordance with the MDAR and ARRIVE reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-490/rc).
Methods
Extraction of YYR
The formula of YYR consists of 7 TCM ingredients, including Astragalus mongholicus (Huang qi), Atractylodes macrocephala (Bai zhu), Paris polyphylla (Dian chong lou), Ligustrum lucidum (Nv zhen zi), Polygonatum sibiricum (Huang jing), Epimedium brevicornu (Ying yang huo), and Dried toadskin (Gan chan pi). These herbs were powdered and mixed (ratio 10:3:5:5:10:10:3), boiled with water repeatedly for eight times within one hour, filtered and concentrated, centrifuged and added with the same volume of ethanol to the super serum, frozen and dried, filtered with a filter to remove bacteria to prepare YYR extract, and stored at 4 ℃. The YYR extracts was mixed with the medium, filtered by filter, and prepared into YYR extracts with concentrations of 1, 0.5, 0.25 mg/mL for experiments.
Cell culture
A549 cell line was acquired from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI)-1640 containing 10% fetal bovine serum (FBS) at 37 ℃ with 5% CO2.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was evaluated using a CCK-8 assay with a Cell Proliferation and Cytotoxicity Assay Kit (CP002; SAB, Sioux Falls, SD, USA). Briefly, 100 mL of cell suspension containing 2×103 A549 cells were plated into a 96-well plate (3×103 cells/mL) for 12 hours, and then incubated with YYR (0–1 mg/mL) for 0-, 24-, 48-, and 72-hour. YYR (0–1 mg/mL) without cell suspension was also set and incubated for 0, 24, 48, and 72 hours. Then, all sample were incubated with 10 µL of CCK-8 reagents for 1 hour, and the cell proliferation was detected using a microplate reader (DNM-9602; Perlong, Nanjing, China) at 450 nm, optical density (OD) values corresponding to unplaced cell suspension holes were removed.
Apoptosis detection
Cells were cultured with YYR of PFK15 for 24 hours prior to being collected and strained with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) double stain (C1063, Beyotime, Nantong, China). According to the manufacturer’s instructions, 5×105–1×106 cells were resuspended in 195 µL Annexin V-FITC binding buffer, following by incubation in 5 µL Annexin V-FITC for 15 minutes at 4 ℃ in the dark. Subsequently, cells were incubated in 5 µL PI for 5 minutes at 4 ℃ in the dark. A tube without incubation of both Annexin V-FITC and PI was used as a control. The apoptotic rate of cells was evaluated using a flow cytometer with a BD AccuriTM C6 Software [Becton, Dickinson, and Co. (BD) Biosciences, San Jose, CA, USA].
Extracellular acidification rate assays
A Seahorse XF96 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA) was used to investigate the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR). Briefly, 1×104 cells per well were seeded into a Seahorse XF 96 cell culture microplate. After baseline evaluation, ECAR determination was carried out, and the glucose, oligomycin, and 2-DG were added into each well at the indicated time points. Then, the OCR was performed by adding the oligomycin and the mitochondrial complex I inhibitor rotenone to the mitochondrial complex III inhibitor antimycin A (Rote/AA). Data were analyzed with the Seahorse XF-96 Wave software. OCR and ECAR were represented in pmol/min and mpH/min, respectively.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with Trizol reagent and reversed transcribed onto complementary DNA (cDNA) using the RevertAid First-Strand cDNA Synthesis kit (Fermentas Co., Hanover, Germany). Subsequently, RT-qPCR assay was performed with a SYBR Green Mix (Thermo Fisher Scientific, Waltham, MA, USA). The primers used in the PCR were: PFKFB3 forward: 5'-TACGCTCTGTTCTCTTTCG-3', reverse: 5'-CTGCCACTCTTATCTTCTGAC-3'; GAPDH forward: 5'-CTGCCCAGAACATCATCC-3', reverse: 5'-CTCAGATGCCTGCTTCAC-3'. The expression levels of messenger RNA (mRNA) were calculated by the 2−ΔΔCT method.
Western blotting
Total proteins were prepared using radioimmunoprecipitation assay (RIPA) lysis buffer, with concentrations analyzed with the bicinchoninic acid (BCA) method. Then, equal amounts of protein were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the target protein bands were blotted onto a nitrocellulose blotting membrane. The blotted membrane was blocked with 5% skimmed milk. After, membranes were successively incubated with primary antibodies (dilution: PFKFB3, 1:1,000; GAPDH, 1:2,000) and horseradish peroxidase (HRP)-conjugated secondary antibodies (dilution: 1:1,000). Finally, the target bands were visualized using enhanced chemiluminescence (ECL) reagents and a Tanon-5200 system (Tanon, Shanghai, China).
Cell transfection
In order to overexpress PFKFB3 in A549 cells, oePFKFB3 was constructed by inserting the PFKFB3 gene fragment into a vector plasmid pcDNA3.1. Lipo2000 (Thermo Fisher) was used to transfect oePFKFB3 into cells following the standard protocols.
Tumorigenicity assay in nude mice
BALB/c athymic nude mice (4–6 weeks old, purchased in the Sino-foreign joint venture Shanghai SIPple Bikai Laboratory Animal Co., Ltd., Shanghai, China) were divided into 4 groups (n=6 per group): vehicle, PFK15, YYR_L, and YYR_H. For all groups, 1×105 A549 cells were injected into either side of the posterior flank of the same female nude mouse. The YYR extracts and PFKFB3 inhibitor PFK15 were dissolved with 0.5% CMC-Na. To the nude mice in the PFK15, YYR_L, and YYR_H groups were administered orally with 5 mg/kg PFK15, 0.64 g YYR, and 1.28 g YYR, respectively. Control groups were treated with an equivalent volume of the vehicle (0.5% CMC-Na). The drug administration was conducted once per day and lasted for 2 weeks. Tumor growth was measured every 3 days. Tumor volume (V) was monitored by measuring the length (L) and width (W) of the tumor with calipers, and was calculated using the formula V = (L × W2)/2. Animal experiments were granted by the Ethics Committee of Shanghai Chest Hospital Affiliated to Shanghai Jiao Tong University [No. (KS (Y) 1804), Shanghai, China] in compliance with Chinese national guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Cancer tissue was fixed in 10% formalin for 48 hours prior to dehydration in graded ethanol. The paraffin sections (6 µm) were cut using a microtome (Leica, Wetzlar, Germany). For the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, sections were incubated with 0.3% Triton X-100 in 0.1 M phosphate-buffered saline (PBS) for 30 minutes and then rinsed with PBS 3 times. Subsequently, these sections were incubated with TUNEL reagents (Beyotime, China) for 1 hour. TUNEL-positive cells were detected using a fluorescence microscope (Leica). TUNEL-positive points are calculated using DAPI and TUNEL positive points through image J software. The positive points of DAPI were calculated as the total number of cells, the positive points of TUNEL were calculated as the tunel points number of cells, the ratio of TUNEL/DAPI × 100% as apoptotic cells was calculated.
Statistical analysis
GraphPad Prism 8.0 software (San Diego, CA, USA) was used for statistical analysis. At least 3 repetitive experiments were performed for each result. Data were expressed as mean ± standard deviation (SD), and comparisons of 3 or more independent groups were performed with a Kruskal-Wallis nonparametric analysis of variance with Dunn’s post hoc test. A P value less than 0.05 was considered significant.
Results
YYR suppressed cell proliferation in A549 cells
We used CCK-8 to detect the effect of YYR on the viability of A549 cells. As shown in Figure 1, the treatment of YYR (1 mg/mL) for 24 hours was able to mildly inhibit the proliferation of A549 cells. However, when the incubating time was extended to 48 and 72 hours, the proliferation of A549 cells was moderately inhibited by different doses of YYR (0.125, 0.25, 0.50, 1.00 mg/mL). The 72 h IC50 (half maximal inhibitory concentration) mortality rate of YYR at 0.5 and 1 mg/mL was more than half, YYR at 0.5 and 1 mg/mL for 48 h was selected as the experimental conditions. In order to investigate whether the effect of YYR on the viability of A549 cells was due to apoptosis and affected the glycolytic pathway, the concentrations of 0.50 and 1 mg/mL, and the treatment time of 48 hours, were selected for the subsequent experiments.

YYR promoted apoptosis while inhibiting energy metabolism and PFKFB3 expression in A549 cells
In order to understand the roles of PFKFB3 in apoptosis and energy metabolism, we treated A549 cells with the PFK15, a PFKFB3 inhibitor. Annexin V-FITC was used to detect apoptotic-inducing abilities of YYR and PFK15 in A549 cells. PFK15 intervention significantly induced the apoptosis of A549 cells. Similar to the results obtained in the CCK-8 experiment, the treatments with YYR (0.5 and 1 mg/mL) induced apoptosis in A549 cells (Figure 2A).
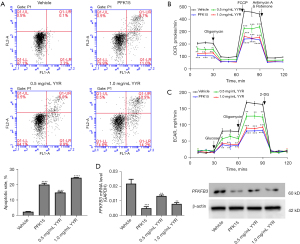
ECAR and OCR assess the mitochondrial function of cells and aims to understand changes in the energetic metabolism of cells. Therefore, we evaluated the effect of YYR on the energy metabolism of cells by detecting the OCR and ECAR in A549 cells. As shown in Figure 2B, YYR (0.5 and 1 mg/mL) and PFK15 were able to significantly inhibit the oxygen consumption of cells. Similarly, the intervention of YYR and PFK15 inhibited the levels of ECAR in cells, compared to the vehicle group (Figure 2C). To further investigate the effects of YYR on the expression of PFKFB3, it was found that, similarly to the PFKFB3 inhibitor, YYR also inhibited the expression of mRNA and PFKFB3 (Figure 2D).
YYR attenuated the overexpression of PFKFB3 mediating apoptosis resistance and energy metabolism in A549 cells
To further elucidate the importance of PFKFB3 in YYR-induced apoptosis and inhibition of energy metabolism, we prepared PFKFB3-overexpressed A549 cells by cell transfection with over expressed PFKFB3 (oePFKFB3). As can be seen from Figure 3, compared with normal cells, both mRNA and protein expression of PFKFB3 in PFKFB3-transfected A549 cells was significantly increased. Furthermore, oePFKFB3 treatment was able to promote cell growth and inhibit apoptosis. However, this situation could be attenuated by YYR treatment (Figure 4A). Similarly, results of ECAR and OCR tests showed that, compared with the vector group, oePFKFB3 treatment improved the oxygen consumption and ECAR of cells while increasing cell metabolism. However, YYR treatment reversed these changes and inhibited the glycolysis process of the cells (Figure 4B,4C). Additionally, YYR was able to inhibit the mRNA and protein overexpression of oePFKFB3 cells (Figure 4D).


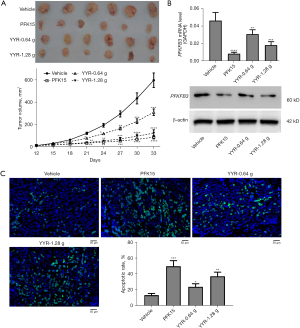
YYR promoted cell apoptosis while inhibiting tumor growth and PFKFB3 expression in vivo
Finally, the A549 cells were subcutaneously inoculated into nude BALB/c mice to confirm the in vivo anti-tumor effect of YYR on lung cancer cells. As shown in Figure 5A, YYR and PFK15 treatments were able to significantly inhibit tumor growth. After mice were sacrificed, tumor tissue was collected for RT-qPCR and western blot analysis. Compared to control tumor tissues, PFK15 significantly inhibited the mRNA and protein expression of PFKFB3. This result was similar to that of treatment with YYR (Figure 5B). In addition, the results of TUNEL assay revealed a significant increase in cell apoptosis in tumor tissues treated with PFK15. This was found to be similar to that of YYR-treated mice (Figure 5C).
Discussion
The morbidity and mortality of lung cancer has been rising (1-4), and currently, the drugs used in the treatment of this disease present significant limitations and adverse reactions (5-8). Therefore, it is necessary to explore new drugs with good efficacy and safety levels against lung cancer.
Chinese herbal medicines have played a crucial role against diseases for thousands of years. Increased evidence on extracts or monomers with promising anti-tumor activity has been found from herbal medicine compounds such as oleanolic acid, emodin, resveratrol, ginsenosides, matrine, and berberine (19-22). Existing studies have proved that some Chinese herbs and monomers have regulatory effects on glucose metabolism of tumor cells, which lays a foundation for our follow-up research on Chinese herbal compounds. For example, as an important anticancer component of Rhizoma japonicum, Zonin I can inhibit the proliferation of hypoxic laryngeal cancer Hep-2 cells and the expression of HIF-1α and VEGF, thereby reducing cell glycolysis and slowing down the generation of pathologic vascular endothelial cells. The Ganoderma steroid extract extracted from Ganoderma lucidum has a high binding affinity for hexokinase 2 (HK2) and acts as a natural enzyme inhibitor to inhibit HK2, which is a rate-limiting enzyme pathway in glycolysis, thereby reducing the rate of cellular glycolysis (23,24). YYR is one of the commonly used prescriptions in the treatment of lung cancer with significant clinical efficacy and safety. It was observed that YYR inhibited the glycolysis of human lung adenocarcinoma cell A549, which may be because the prescription controlled the energy uptake of lung adenocarcinoma cell by inhibiting the expression of HIF-1α and PFKFB3 in the glycolysis pathway of lung adenocarcinoma cell, thus inhibiting its proliferation process. It also could inhibit the migration and invasion of lung cancer cells and down-regulate the expression of angiogenesis-related factors, such as VEGF and HIF-1α (17,18). Apoptosis is one of the main death pathways of cells and plays a key role in cell death and proliferation, maintaining the homeostasis. Over- or under-regulation of apoptosis results in diseases such as tumors, rheumatoid arthritis, and cardiovascular diseases (25). The tumor/cancer development is closely related to the uncontrolled cell proliferation and inadequate cell apoptosis. So, pro-apoptosis drugs are used for treating or controlling tumors (26). In this study, we report that RPL6 can be used as a preventive biomarker and potential therapeutic for lung carcinoma treatment and prognosis by accommodating the AKT signaling pathway (27). Hypoxia is one of the basic characteristics of the solid tumor microenvironment. When the tumor grows to more than 1mm3, a considerable part of the cells in the tumor are in a state of hypoxia. The energy metabolism of normal tissue cells in aerobic environment is mainly aerobic oxidation, and when oxygen supply is insufficient, glycolysis provides the main energy. In the process of hypoxia in the tumor microenvironment and self-adaptation to hypoxia, the energy metabolism structure of tumor cells has undergone great changes, that is, regardless of normal partial pressure of oxygen or hypoxia, the glycolysis pathway is preferred to provide energy, which is also known as the Warburg effect (28). Compared with normal tissues, PFKFB3 is overexpressed in tumors, leading to the accumulation of fructose-2,6-bisphosphate and heterogeneous activation of phosphofructokinase (PFK), promoting a more intensive glycolysis (29). Therefore, PFKFB3 is considered one of the key enzymes in cell glycolysis, playing an important role in the Warburg effect in tumor cells (30). Furthermore, previous studies have found that high levels of PFKFB3 mRNA and its transcriptional protein can also inhibit the growth of a variety of tumor cells and induce apoptosis (31-34). In this study, we found that the TCM formula YYR exhibited pro-apoptotic and anti-glycolytic effects, and the PFKFB3 might be a potential drug target for YYR.
Conclusions
Collectively, our study showed that YYR was able to induce apoptosis of A549 cells, reduce ECAR, and inhibit cell metabolism. Additionally, YYR was shown to have a regulatory effect on mRNA and protein expressions of PFKFB3. We demonstrated that YYR could promote lung cancer cell apoptosis and inhibit energy metabolism by inhibiting the expression of PFKFB3. We also suggest that YYR may be a suitable supplement or alternative drug against lung cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-490/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-490/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-490/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-490/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were granted by the Ethics Committee of Shanghai Chest Hospital Affiliated to Shanghai Jiao Tong University [No. (KS (Y) 1804), Shanghai, China] in compliance with Chinese national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Jakobsen E, Rasmussen TR, Green A. Mortality and survival of lung cancer in Denmark: Results from the Danish Lung Cancer Group 2000-2012. Acta Oncol 2016;55:2-9. [Crossref] [PubMed]
- Li J, Guo W, Ran J, et al. Five-year lung cancer mortality risk analysis and topography in Xuan Wei: a spatiotemporal correlation analysis. BMC Public Health 2019;19:173. [Crossref] [PubMed]
- Dean EJ, Ward T, Pinilla C, et al. A small molecule inhibitor of XIAP induces apoptosis and synergises with vinorelbine and cisplatin in NSCLC. Br J Cancer 2010;102:97-103. [Crossref] [PubMed]
- Bartucci M, Svensson S, Romania P, et al. Therapeutic targeting of Chk1 in NSCLC stem cells during chemotherapy. Cell Death Differ 2012;19:768-78. [Crossref] [PubMed]
- Roach C, Zhang N, Corigliano E, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non-Small-cell Lung Cancer. Appl Immunohistochem Mol Morphol 2016;24:392-7. [Crossref] [PubMed]
- Ruiz-Martinez M, Navarro A, Marrades RM, et al. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget 2016;7:51515-24. [Crossref] [PubMed]
- Tan AC, Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol 2022;40:611-25. [Crossref] [PubMed]
- Hayashi H, Nadal E, Gray JE, et al. Overall Treatment Strategy for Patients With Metastatic NSCLC With Activating EGFR Mutations. Clin Lung Cancer 2022;23:e69-82. [Crossref] [PubMed]
- Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med 1999;5:662-8. [Crossref] [PubMed]
- Quintanal-Villalonga Á, Mediano M, Ferrer I, et al. Histology-dependent prognostic role of pERK and p53 protein levels in early-stage non-small cell lung cancer. Oncotarget 2018;9:19945-60. [Crossref] [PubMed]
- Butkiewicz D, Rusin M, Enewold L, et al. Genetic polymorphisms in DNA repair genes and risk of lung cancer. Carcinogenesis 2001;22:593-7. [Crossref] [PubMed]
- WARBURG O. On the origin of cancer cells. Science 1956;123:309-14. [Crossref] [PubMed]
- Shi WK, Zhu XD, Wang CH, et al. PFKFB3 blockade inhibits hepatocellular carcinoma growth by impairing DNA repair through AKT. Cell Death Dis 2018;9:428. [Crossref] [PubMed]
- Xiang Y, Zhang M, Xu ZH. A randomized controlled study on the clinical effect of Yiqi Yangjing method in the treatment of advanced non-small cell lung cancer in the elderly patients. J New Chin Med 2012;44:84-6.
- Yan GY. Effect of Yiqi Yangjingdecoction on the improvement of cancerous fatigue of elderly patients with non-small cell lung cancer after interventional therapy. Prac J Cancer 2018;33:588-91.
- Zhang M, Dong Y, Yang X, et al. Effect of Yiqi Yangjing Prescription on lung cancer cells via Apelin/APJ signaling pathway. Chin Med 2019;14:698-702.
- Zhang JB, Chen C, Tao ZZ. Plant extract anti-cancer research progress. J Int Oncol 2012;39:579-84.
- Peng W, Qin R, Li X, et al. Polygonum cuspidatum Sieb. et Zucc.: a review of its botany, phytochemistry, pharmacology, and potential applications. J Ethnopharmacol 2013;148:729-45. [Crossref] [PubMed]
- Wang H, Zhong W, Zhao J, et al. Oleanolic Acid Inhibits Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma by Promoting iNOS Dimerization. Mol Cancer Ther 2019;18:62-74. [Crossref] [PubMed]
- Sun M, Ye Y, Xiao L, et al. Anticancer effects of ginsenoside Rg3 Int J Mol Med 2017;39:507-18. (Review). [Crossref] [PubMed]
- Deng BF, Liao M, Qiu RM, et al. Effects of bilonin I on proliferation of Hep-2 cells and expression of HIF-1αand VEGF in hypoxic laryngeal carcinoma. Journal of Anhui Medical University 2016;1613-17.
- Bao F, Yang K, Wu C, et al. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018;125:123-9. [Crossref] [PubMed]
- Zhang Q, Duan HX, Li RL, et al. Inducing Apoptosis and Suppressing Inflammatory Reactions in Synovial Fibroblasts are Two Important Ways for Guizhi-Shaoyao-Zhimu Decoction Against Rheumatoid Arthritis. J Inflamm Res 2021;14:217-36. [Crossref] [PubMed]
- Arumugam A, Abdull Razis AF. Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: a Review. Asian Pac J Cancer Prev 2018;19:1439-48. [PubMed]
- Zhang J, Ma Q, Han Y, et al. Downregulated RPL6 inhibits lung cancer cell proliferation and migration and promotes cell apoptosis by regulating the AKT signaling pathway. J Thorac Dis 2022;14:507-14. [Crossref] [PubMed]
- Siska PJ, Singer K, Evert K, et al. The immunological Warburg effect: Can a metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy? Immunol Rev 2020;295:187-202. [Crossref] [PubMed]
- Ros S, Schulze A. Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism. Cancer Metab 2013;1:8. [Crossref] [PubMed]
- Minchenko OH, Tsuchihara K, Minchenko DO, et al. Mechanisms of regulation of PFKFB expression in pancreatic and gastric cancer cells. World J Gastroenterol 2014;20:13705-17. [Crossref] [PubMed]
- Lu L, Chen Y, Zhu Y. The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget 2017;8:62793-802. [Crossref] [PubMed]
- O'Neal J, Clem A, Reynolds L, et al. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and the growth of HER2+ breast cancer. Breast Cancer Res Treat 2016;160:29-40. [Crossref] [PubMed]
- Hu KY. Targeting of MCT1 and PFKFB3 influences cell proliferation and apoptosis in bladder cancer by altering the tumor microenvironment. Oncol Rep 2016;36:945-51. [Crossref] [PubMed]
- Zhu W, Ye L, Zhang J, et al. PFK15, a Small Molecule Inhibitor of PFKFB3, Induces Cell Cycle Arrest, Apoptosis and Inhibits Invasion in Gastric Cancer. PLoS One 2016;11:e0163768. [Crossref] [PubMed]
(English Language Editor: J. Jones)


