
Predictive value of serum β2-microglobulin in cardiac valve calcification in maintenance hemodialysis patients
Highlight box
Key findings
• Serum β2-microglobulin (β2-MG) in maintenance hemodialysis (MHD) patients has a positive correlation with the severity of cardiac valve calcification (CVC) and can predict the occurrence of CVC.
What is known and what is new?
• Many researchers have reported on the risk factor analysis of CVC in MHD patients.
• There are very few reports on the relationship between serum β2-MG and CVC. We speculate that elevated β2-MG may promote the development of CVC. For elderly patients with MHD and high β2-MG levels, it is critical to have a high suspicion of the occurrence of CVC.
What is the implication, and what should change now?
• This study showed significant positive correlation of β2-MG with cardiac valvular calcification which contributes to significant mortality and morbidity in end-stage renal disease (ESRD) patients. More prospective studies will help establish this association and could potentially provide an interventional tool to tackle this important problem in ESRD patients.
Introduction
In recent years, the incidence of chronic kidney disease (CKD) has increased year by year, and the number of CKD patients progressing to end-stage renal disease (ESRD) has been increasing. The primary renal replacement therapy in patients with ESRD is hemodialysis (HD). A study has shown that 50% of MHD patients die of cardiovascular disease (CVD) (1). Cardiac valve calcification (CVC) is an independent predictor of adverse cardiovascular events (2-4). In patients with CKD, valvular calcification is considered a poor prognostic factor (5). In patients requiring renal replacement therapy, cardiac valve disease occurs 10 times faster than in patients with preserved renal function (6). A recent meta-analysis highlighted the strong association of CVC with higher cardiovascular and all-cause mortality in dialysis patients (7). A study has also shown that perivalvular regurgitation and heart conduction abnormalities are closely related to CVC (8). Therefore, improving understanding of CVC risk factors may facilitate earlier intervention. Many researchers have reported on the risk analysis of CVC in maintenance HD (MHD) patients. A recent review (6) showed that the factors leading to CVC are: (I) metabolic factors and inflammation; (II) mineral and hormone-related factors, including hypercalcemia, hyperphosphatemia, hypomagnesemia, metabolic alkalosis, hyperparathyroidism, elevated fibroblast growth factor-23 and sclerostin; (III) drugs, such as calcium supplements and warfarin. β2-MG is a low molecular weight protein associated with major histocompatibility complex (MHC) class I molecules, mainly cleared by the kidney, hence elevated β2-MG concentrations in the CKD population. Meanwhile, β2-MG can act as an inflammatory factor and has been extensively studied in peripheral arterial disease and CVD (9-11). However, there are very few reports on the relationship between serum β2-microglobulin (β2-MG) and CVC. This study is designed to explore the relationship of β2-MG with that of CVC in MHD patients. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1185/rc).
Methods
Patients
Patients who underwent MHD for more than 3 months in the Department of Nephrology of First People’s Hospital of Nantong City from November 2012 to November 2019, and had a complete follow-up data set were chosen as the study participants. All patients had no history of peritoneal dialysis. Echocardiography (echo) was conducted ≥3 months after the first dialysis. The exclusion criteria for the study were as follows: (I) serious cardiovascular and cerebrovascular events that had occurred within 3 months; (II) history of malignancy; (III) acute or chronic infection within the last month; (IV) severe arrhythmia and heart failure within the last month; (V) people with autoimmune diseases; (VI) history of glucocorticoid and immunosuppressive medication within 6 months; (VII) history of warfarin within 6 months; (VIII) patients with a history of blood transfusion within the recent 3 months (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First People’s Hospital of Nantong City (No. 2019KW008) and individual consent for this retrospective analysis was waived.
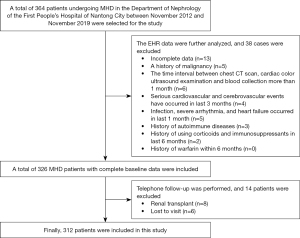
Grouping
All patients underwent routine echo. On echo, CVC was defined as a bright echo with a diameter greater than 1 mm in the aortic valve, mitral valve cusp, or mitral valve annulus (12-14). The patients were then divided into the CVC group and the non-CVC group according to the presence or absence of CVC.
Dialysis program
The dialysis frequency of the enrolled patients in the past three months was 3 times/week, 4 hours each time, using bicarbonate dialysate, polysulfone membrane dialyzer (area 1.5 m2; the clearance rates of BUN and creatinine were 269 vs. 238 mL/min, respectively). According to the Chinese standard operating procedures for blood purification, a dialysate flow rate of 500 mL/min, and a blood flow rate of 200–400 mL/min. The dialysis mode was HD. During dialysis, all enrolled patients were anticoagulated with low molecular weight heparin calcium. According to China’s 2021 Blood Purification Standard Operating Procedure (SOP), we believe that the goal for dialysis adequacy is HD adequacy (Kt/V urea) >1.2 and urea reduction ratio (URR) >65%. Kt/V urea = −Ln(R − 0.008 × dialysis time) + (4 − 3.5R) × (ΔBW/BW), Ln is natural logarithm, the unit of dialysis time is hour; R is post dialysis blood urea/pre dialysis blood urea, ΔBW is ultrafiltration volume (L); BW is the body weight (kg). URR (%) = (C0 − C)/C0 × 100%, where C0 represents the blood urea concentration before dialysis and C represents the blood urea concentration after dialysis.
Data collection
The clinical data of all participants were recorded, including age, gender, primary disease, medication history [including calcium-based phosphate binder (CPB), non-CPB (NCPB), active vitamin D, and statin], body mass index (BMI), HD vintage, Kt/V urea, URR, predialysis systolic blood pressure (SBP), predialysis diastolic blood pressure (DBP), hemoglobin (Hb), albumin (Alb), blood glucose (Glu), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), corrected calcium (Ca), serum inorganic phosphorus (Pi), serum magnesium (Mg), high-sensitivity C-reactive protein (hs-CRP), parathyroid hormone (PTH), serum β2-MG, 25-hydroxyactive vitamin D3 [25(OH)VitD3], alkaline phosphatase (AKP), and N-terminal precursor brain natriuretic peptide (NT-proBNP). Cardiac ultrasound results were recorded, including the presence or absence of CVC, the location of CVC, ejection fraction (EF) value, and left ventricular mass index (LVMI). The dialysis adequacy indicators Kt/V urea and URR are evaluated once every three months on average, and the data we collected are the average of the two evaluations. For all biochemical indicators, the results of fasting blood collection before the second weekly dialysis were selected. Care was taken that the interval between blood collection time and cardiac ultrasound examination should not exceed 1 month.
Echo is the gold standard for detecting heart valve disease (15). Echo was performed by cardiac ultrasound specialists at our hospital, using Philips EPIQ 7C (Philips Healthcare, Andover, MA, USA), IE33 echocardiograph, X5-1 probe/frequency 1–3 MHz, and S5-1 probe/frequency 1–5 MHz.
Statistical analysis
The software packages SPSS 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) were used for statistical processing of data. The normally distributed measurement data were expressed as mean ± standard deviation (SD), and the comparison between groups was tested by analysis of variance (ANOVA). NT-proBNP had non-normally distributed data and was converted to normal distribution data by logarithmic (Lg) before analysis. Moreover, variables HD vintage and PTH had non-normally distributed data, and were expressed as median (interquartile range), and a nonparametric test was used to compare groups. Categorical data were expressed as percentages or frequencies, and comparisons between groups were performed using the chi-squared test. Relevant risk factors were analyzed by binary logistic regression analysis. The receiver operating characteristic curve (ROC) and Youden index were used to evaluate the prediction of β2-MG on heart valve calcification in MHD patients, and the cut-off value was calculated. A decision tree (DT) model was used to identify important determinants for predicting the development of CVC in patients with MHD. A difference with P<0.05 was considered statistically significant.
Results
Baseline information
A total of 312 MHD patients were enrolled in this study, with a mean age of (60.1±14.9) years, including 174 males (55.8%) and 138 females (44.2%). There were 107 cases (34.3%) in the CVC group and 205 cases (65.7%) in the non-CVC group. The proportion of males, and mean values of SBP, DBP, Pi, and Kt/V urea in the CVC group were lower than those in the non-CVC group. The proportion of diabetic nephropathy, mean values of age, Ca, hs-CRP, and β2-MG, and median value of PTH in the CVC group were significantly higher than those in the non-CVC group (P<0.05; Table 1).
Table 1
| Characteristics | Total populations (n=312) | CVC group (n=107) | Non-CVC group (n=205) | t/z/χ2 | P value |
|---|---|---|---|---|---|
| Gender (male) | 174 (55.8) | 51 (47.7) | 123 (60.0) | 4.338 | 0.037 |
| Age (years) | 60.1±14.9 | 70.62±9.18 | 54.61±14.37 | 10.456 | <0.001 |
| BMI (kg/m2) | 23.85±4.06 | 24.09±4.32 | 23.72±3.92 | 0.719 | 0.473 |
| SBP (mmHg) | 152.99±24.03 | 149.26±23.82 | 154.91±23.87 | −1.974 | 0.049 |
| DBP (mmHg) | 83.16±14.89 | 77.03±11.49 | 86.34±15.48 | −5.464 | <0.001 |
| HD vintage (months) | 3 [3, 17] | 5 [3, 20] | 3 [3, 15.5] | −1.811 | 0.070 |
| Medication history | |||||
| CPB | 20 (6.4) | 5 (4.6) | 15 (7.3) | 0.819 | 0.365 |
| NCPB | 24 (7.7) | 9 (8.4) | 15 (7.3) | 0.119 | 0.731 |
| Active vitamin D | 133 (42.6) | 45 (42.1) | 88 (42.9) | 0.022 | 0.883 |
| Statin | 62 (19.9) | 27 (25.2) | 35 (17.1) | 2.940 | 0.086 |
| Primary disease | 25.958 | <0.001 | |||
| CGN | 116 (37.2) | 18 (16.8) | 98 (47.8) | ||
| DN | 107 (34.3) | 52 (48.6) | 55 (26.8) | ||
| Others | 89 (28.5) | 37 (34.6) | 52 (25.4) | ||
| Hb (g/L) | 91.3±22.37 | 93.02±23.24 | 90.41±21.90 | 0.978 | 0.329 |
| Alb (g/L) | 34.69±5.62 | 34.24±4.89 | 34.93±5.96 | −1.019 | 0.309 |
| Glu (mmol/L) | 5.46±1.51 | 5.56±1.62 | 5.41±1.45 | 0.836 | 0.404 |
| TC (mmol/L) | 4.07±1.13 | 4.04±1.07 | 4.08±1.17 | −0.323 | 0.747 |
| Ca (mmol/L) | 2.17±0.26 | 2.21±0.21 | 2.14±0.28 | 2.168 | 0.031 |
| Pi (mmol/L) | 1.76±0.57 | 1.62±0.46 | 1.84±0.60 | −3.276 | 0.001 |
| Mg (mmol/L) | 1.06±0.26 | 1.08±0.24 | 1.05±0.27 | 0.847 | 0.397 |
| hs-CRP (mg/L) | 23.47±11.28 | 26.71±13.97 | 21.77±9.18 | 0.374 | <0.001 |
| PTH (pg/mL) | 261.15 [151.68, 455.58] | 266.1 [140.55, 465.95] | 210.85 [127.05, 335.93] | −3.163 | 0.002 |
| Lg(NT-proBNP) | 4.08±0.56 | 4.14±0.44 | 4.04±0.62 | 1.361 | 0.175 |
| LVMI (g/m2) | 149.04±36.61 | 152.0±38.18 | 147.50±35.77 | 0.992 | 0.322 |
| 25(OH)VitD3 (ng/mL) | 12.3±8.79 | 11.54±8.51 | 12.74±8.95 | −1.054 | 0.293 |
| β2-MG (μg/L) | 27.63±11.71 | 33.43±11.77 | 24.61±10.50 | 6.750 | <0.001 |
| Kt/V urea | 1.34±0.27 | 1.29±0.24 | 1.36±0.28 | −1.977 | 0.048 |
| URR (%) | 66.32±7.82 | 65.39±8.36 | 66.79±7.52 | −1.296 | 0.196 |
| AKP (U/L) | 81.11±48.56 | 81.07±40.49 | 81.14±52.43 | −0.013 | 0.990 |
Data are presented as n (%), mean ± SD, or median [IQR]. Kt/V urea = −Ln(R − 0.008 × dialysis time) + (4 − 3.5R) × (ΔBW/BW), Ln is natural logarithm, the unit of dialysis time is hour; R is post dialysis blood urea/pre dialysis blood urea, ΔBW is ultrafiltration volume (L); BW is the body weight (kg). URR (%) = (C0 − C)/C0 × 100%, where C0 represents the blood urea concentration before dialysis and C represents the blood urea concentration after dialysis. CVC, cardiac valve calcification; MHD, maintenance hemodialysis; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HD, hemodialysis; CPB, calcium-based phosphate binder; NCPB, non-CPB; CGN, chronic glomerulonephritis; DN, diabetic nephropathy; Hb, hemoglobin; Alb, albumin; Glu, blood glucose; TC, total cholesterol; Ca, corrected calcium; Pi, inorganic phosphorus; Mg, magnesium; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; Lg, logarithmic; NT-proBNP, N-terminal precursor brain natriuretic peptide; LVMI, left ventricular mass index; 25(OH)VitD3, 25-hydroxyactive vitamin D3; β2-MG, β2-microglobulin; Kt/V urea, HD adequacy; URR, urea reduction rate; AKP, alkaline phosphatase; SD, standard deviation; IQR, interquartile range.
Risk factors for CVC in MHD patients
Univariate analysis showed that gender, age, DBP, Ca, Pi, hs-CRP, and β2-MG were risk factors for CVC in MHD patients. Factors with P≤1 in the univariate analysis were included in the multivariate analysis. And the model included factors such as age, gender, SBP, DBP, HD vintage, Ca, Pi, hs-CRP, PTH, β2-MG, and Kt/V urea. The results showed that age, gender, β2-MG, and Kt/V urea are independent risk factors for heart valve calcification (P<0.05; Tables 2,3).
Table 2
| Characteristics | B | SE | Wald | Df | Sig. | Exp(B) | HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Low | Up | |||||||
| Gender (male) | −0.499 | 0.240 | 4.309 | 1 | 0.038 | 0.607 | 0.379 | 0.973 |
| Age (years) | 0.109 | 0.014 | 60.611 | 1 | <0.001 | 1.115 | 1.085 | 1.146 |
| BMI (kg/m2) | 0.022 | 0.030 | 0.519 | 1 | 0.471 | 1.022 | 0.963 | 1.085 |
| HD vintage (months) | 0.008 | 0.005 | 2.709 | 1 | 0.100 | 1.008 | 0.999 | 1.017 |
| SBP (mmHg) | −0.010 | 0.005 | 3.828 | 1 | 0.050 | 0.990 | 0.980 | >0.999 |
| DBP (mmHg) | −0.050 | 0.010 | 25.021 | 1 | <0.001 | 0.951 | 0.932 | 0.970 |
| Hb (g/L) | −0.002 | 0.006 | 0.116 | 1 | 0.734 | 0.998 | 0.985 | 1.010 |
| Alb (g/L) | −0.022 | 0.021 | 1.039 | 1 | 0.308 | 0.979 | 0.938 | 1.020 |
| Glu (mmol/L) | 0.065 | 0.078 | 0.699 | 1 | 0.403 | 1.068 | 0.916 | 1.245 |
| TC (mmol/L) | −0.035 | 0.108 | 0.105 | 1 | 0.746 | 0.966 | 0.782 | 1.192 |
| Ca (mmol/L) | 1.128 | 0.526 | 4.600 | 1 | 0.032 | 3.088 | 1.102 | 8.653 |
| Pi (mmol/L) | −0.750 | 0.236 | 10.103 | 1 | 0.001 | 0.472 | 0.297 | 0.750 |
| Mg (mmol/L) | 0.381 | 0.451 | 0.714 | 1 | 0.398 | 1.464 | 0.604 | 3.547 |
| hs-CRP (mg/L) | 0.038 | 0.011 | 12.423 | 1 | <0.001 | 1.039 | 1.017 | 1.061 |
| PTH (pg/mL) | −0.001 | 0.000 | 3.654 | 1 | 0.056 | 0.999 | 0.998 | >0.999 |
| Lg(NT-proBNP) | 0.349 | 0.259 | 1.825 | 1 | 0.177 | 1.418 | 0.854 | 2.354 |
| EF (%) | −0.010 | 0.013 | 0.640 | 1 | 0.424 | 0.990 | 0.965 | 1.015 |
| LVMI (g/m2) | 0.003 | 0.003 | 0.982 | 1 | 0.322 | 1.003 | 0.997 | 1.010 |
| β2-MG (μg/L) | 0.070 | 0.012 | 34.554 | 1 | <0.001 | 1.072 | 1.048 | 1.097 |
| URR (%) | −0.023 | 0.018 | 1.660 | 1 | 0.198 | 0.978 | 0.944 | 1.012 |
| Kt/V urea | −0.965 | 0.504 | 3.661 | 1 | 0.056 | 0.381 | 0.142 | 1.024 |
| AKP (U/L) | 0.000 | 0.002 | 0.000 | 1 | 0.990 | 1.000 | 0.995 | 1.005 |
| 25(OH)VitD3 (ng/mL) | −0.016 | 0.015 | 1.106 | 1 | 0.293 | 0.984 | 0.955 | 1.014 |
CVC, cardiac valve calcification; MHD, maintenance hemodialysis; SE, standard error; df, degrees of freedom; sig., significant; HR, hazard ratio; CI, confidence interval; BMI, body mass index; HD, hemodialysis; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hb, hemoglobin; Alb, albumin; Glu, blood glucose; TC, total cholesterol; Ca, corrected calcium; Pi, inorganic phosphorus; Mg, magnesium; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; Lg, logarithmic; NT-proBNP, N-terminal precursor brain natriuretic peptide; EF, ejection fraction; LVMI, left ventricular mass index; β2-MG, β2-microglobulin; URR, urea reduction rate; Kt/V urea, HD adequacy; AKP, alkaline phosphatase; 25(OH)VitD3, 25-hydroxyactive vitamin D3.
Table 3
| Characteristics | B | SE | Wald | Df | Sig. | Exp(B) | HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Low | Up | |||||||
| Gender (male) | 0.964 | 0.455 | 4.482 | 1 | 0.034 | 2.621 | 1.074 | 6.398 |
| Age (years) | 0.097 | 0.018 | 27.714 | 1 | <0.001 | 1.101 | 1.062 | 1.142 |
| SBP (mmHg) | −0.004 | 0.009 | 0.226 | 1 | 0.634 | 0.996 | 0.978 | 1.014 |
| DBP (mmHg) | −0.012 | 0.018 | 0.447 | 1 | 0.504 | 0.988 | 0.955 | 1.023 |
| HD vintage (months) | 0.007 | 0.009 | 0.605 | 1 | 0.437 | 1.007 | 0.99 | 1.024 |
| Ca (mmol/L) | 0.684 | 0.93 | 0.54 | 1 | 0.462 | 1.981 | 0.32 | 12.27 |
| Pi (mmol/L) | −0.173 | 0.387 | 0.2 | 1 | 0.655 | 0.841 | 0.394 | 1.796 |
| hs-CRP (mg/L) | 0.006 | 0.016 | 0.147 | 1 | 0.701 | 1.006 | 0.975 | 1.038 |
| PTH (pg/mL) | 0 | 0.001 | 0.149 | 1 | 0.7 | 1 | 0.999 | 1.001 |
| Kt/V urea | −2.236 | 0.993 | 5.065 | 1 | 0.024 | 0.107 | 0.015 | 0.749 |
| β2-MG (μg/L) | 0.051 | 0.018 | 7.894 | 1 | 0.005 | 1.053 | 1.016 | 1.091 |
Model: included the results of P≤1 in the univariate binary logistic analysis into the multivariate binary logistic analysis. CVC, cardiac valve calcification; MHD, maintenance hemodialysis; SE, standard error; df, degrees of freedom; sig., significant; HR, hazard ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; HD, hemodialysis; Ca, corrected calcium; Pi, inorganic phosphorus; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; Kt/V urea, HD adequacy; β2-MG, β2-microglobulin.
Comparison of β2-MG concentrations in CVC at different sites in MHD patients
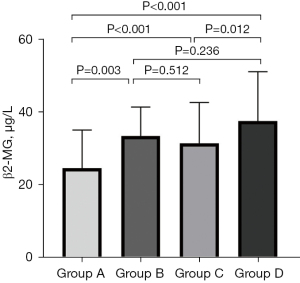
To compare the expression of β2-MG in CVC at different sites, we divided 312 MHD patients into four groups. Group A was without CVC, group B was only with MVC, group C was only with AVC, and group D was with both MVC and AVC. The results of the ANOVA tests showed that there were significant differences among the four groups (P<0.001). Moreover, the concentration of β2-MG in groups B, C, and D was significantly higher than that in group A (P<0.05); whereas the concentration of β2-MG in group D was significantly higher than that in group C (P<0.05; Table 4, Figure 2). Upon performing a Spearman correlation analysis between β2-MG and the number of CVC sites, the final output was: r=0.358 (P<0.001) (the absence of CVC was defined as 0, the presence of only MVC or AVC as 1, and the presence of both MVC and AVC as 2). The results showed a weakly positive correlation between the concentration of β2-MG and the number of CVC.
Table 4
| Characteristic | Group A | Group B | Group C | Group D | P value |
|---|---|---|---|---|---|
| β2-MG (μg/L) | 24.61±10.50 | 33.47±7.96 | 31.42±11.24 | 37.55±13.56 | <0.001 |
Data are presented as mean ± SD. Group A: no calcification; group B: only mitral valve calcification; group C: only AVC; group D: both mitral valve and AVC. β2-MG, β2-microglobulin; CVC, cardiac valve calcification; MHD, maintenance hemodialysis; SD, standard deviation; AVC, aortic valve calcification.
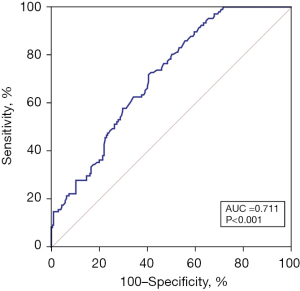
The value of serum β2-MG in predicting CVC in MHD patients
The ROC curve was used to investigate the predictive value of CVC serum β2-MG levels in MHD patients, and a β2-MG cut-off value was determined. When the cut-off level was 25 µg/L, the sensitivity and specificity obtained were 71.96% and 59.51%, respectively (Figure 3).
Binary logistic regression analysis showed that age, gender, β2-MG, and Kt/V urea were independent predictors for CVC in MHD patients (Table 3). As per the cut-off value, the participants were divided into a β2-MG ≥25 µg/L group and β2-MG <25 µg/L group. Using the occurrence of CVC ≥1 as the dependent variable, binary logistic regression analysis revealed the following: uncorrected, the risk of CVC in the higher β2-MG group was 3.80 compared with the lower β2-MG group times [95% confidence interval (CI), 2.28–6.32; P<0.001]. In a model adjusted for gender, age, and Kt/V urea, the higher β2-MG group had a 3.39-fold higher risk of developing CVC compared with the lower β2-MG group (95% CI, 1.63–7.06; P=0.001; Table 5).
Table 5
| Projects | Serum β2-MG levels (μg/L) | P value | |
|---|---|---|---|
| <25 (n=149) | ≥25 (n=163) | ||
| Patients with CVC ≥1 (%) | 29 (19.5) | 78 (47.9) | <0.001 |
| Unadjusted ORs (95% CI) | Reference | 3.80 (2.28–6.32) | <0.001 |
| Multilevel adjusted ORs (95% CI) | |||
| Gender + age + Kt/V urea | Reference | 3.39 (1.63–7.06) | 0.001 |
OR, odds ratio; CI, confidence interval; CVC, cardiac valve calcification; CVC ≥1, CVC occurred in at least 1 site; MHD, maintenance hemodialysis; β2-MG, β2-microglobulin; Kt/V urea, HD adequacy.
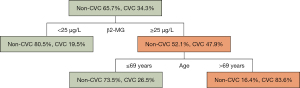
DT model identifies patients at high risk of CVC
In the DT model, the optimal cut-off value of serum β2-MG ≥25 µg/L was determined as the variable for the initial split, and 19.5% of patients with serum β2-MG <25 µg/L developed CVC. Among the patients with serum β2-MG ≥25 µg/L, 47.9% of patients developed CVC. In patients with serum β2-MG ≥25 µg/L, age was identified as a variable for the second split, with the optimal cut-off of >69 years. CVC occurred in 83.6% of patients aged >69 years, whereas only 26.5% of patients aged ≤69 years developed CVC. As per the classification of the DT model, two groups of patients at high risk of CVC were identified (Figure 4).
Discussion
In MHD patients, the presence of CVC is found to increase the risk of cardiovascular death by 5.4 times (14). Studies have shown that the occurrence of CVC is an active and adjustable biological process involving oxidative stress, inflammatory response, lipid deposition, bone metabolism, and other pathways, all of which can lead to vascular damage and activate multiple signaling pathways to promote the occurrence and progression of vascular calcification (16-19). In our study, the CVC group had higher HD vintage and lower dialysis adequacy. It shows that the higher the HD vintage, the worse the dialysis adequacy and the more prone to CVC. Therefore, in order to delay the occurrence of CVC, we need to improve patients’ dialysis adequacy and find other factors that affect CVC and intervene.
β2-MG is an 11.8 kDa glycosylated polypeptide present in all nucleated cells (20). β2 -MG is widely found in blood plasma, urine, cerebrospinal fluid, saliva, and colostrum. Under normal conditions, almost all β2-MG is filtered through glomeruli, 99% is reabsorbed in renal tubules and only slightly excreted in urine. With the renal hypofunction, β2-MG gradually decreased through glomerular filtration, and the blood β2-MG concentration gradually increased. In the MHD population, medium molecular toxins such as β2-MG cannot be effectively cleared by ordinary HD, and blood β2-MG constantly accumulate in the body with the gradual decline of residual renal function. As a new potential inflammatory factor (21,22), β2-MG has been extensively studied in autoimmune diseases, malignancies, kidney diseases, peripheral arterial disease, and CVDs in recent years (9-11). As we all know, hs-CRP is also an indicator of response inflammation. In this study, hs-CRP and β2-MG in the CVC group were significantly higher than those in the non-CVC group. It shows that CVC patients are more likely to be in a state of micro-inflammation. β2-MG is abundantly present on the surface of lymphocytes and monocytes. It is synthesized in large quantities by lymphocytes and is regulated by interferon and monocytes (23). The infiltrated areas of the heart valve often appear in sites where vascular endothelial cells are activated and express adhesion molecules that promote recruitment of monocytes and macrophages and transendothelial migration into the valve. Atherosclerosis is a chronic inflammatory process (24), and certain stimuli promote the development of atherosclerosis by persistent inflammatory stimulations (25). Jadoul et al. found that approximately 90% of MHD patients who have been on dialysis for more than 7 years can develop β2-MG amyloid deposition, and the pathological distribution of amyloid deposition is similar to the sites of tissue calcification (26). Mitral valve calcification can cause mitral regurgitation and high left atrial pressure, pulmonary hypertension, left ventricular (LV) dilatation, and eccentric hypertrophy. When mitral valve calcification leads to mitral valve stenosis, heart failure such as chest tightness, shortness of breath, and difficulty breathing may occur. Similarly, aortic stenosis can occur after aortic valve calcification, leading to LV remodeling, hypertrophy and dysfunction.
The results of the present study showed that the level of β2-MG in MHD patients in the CVC group was significantly higher than that in the non-CVC group. The univariate and multivariate binary logistic regression analyses showed that β2-MG was an important independent risk factor for CVC in MHD patients. Furthermore, the risk ratio of CVC was significantly increased in patients with high β2-MG levels. Even after adjusting for age, gender, and Kt/V urea, the risk of CVC in patients with high β2-MG levels was 3.39 times that of the low β2-MG level group. Therefore, this study concluded that the increased level of β2-MG may play an important role in the pathophysiological process of CVC in MHD patients, and the mechanism may be closely related to the micro-inflammatory state and the potential inflammatory effect of β2-MG in MHD patients.
Ikee et al. (13,27) reported that there is correlation between serum β2-MG and CVC, and β2-MG concentration is associated with the number of CVC. Our research reached the same conclusion. Furthermore, their findings showed that β2-MG was associated with MVC but not AVC. In our study, the concentration of β2-MG was significantly higher in the group with AVC or MVC than in the group without CVC, and the concentration of β2-MG gradually increased as the number of CVC increased. The findings implied that β2-MG may be involved in the formation of CVC.
Furthermore, based on the significant risk factors that influence the occurrence of CVC, this study developed a DT model to identify patients at high risk of CVC. For high-risk patients, the likelihood of developing CVC is approximately 83.6%. That is, for elderly patients with MHD and high β2-MG levels, it is critical to have a high suspicion of the occurrence of CVC. When we discover the high-risk factors of CVC, we need to take measures to delay the occurrence of CVC as much as possible. For example, use low-calcium dialysate, increase the adequacy of dialysis, replace high-flux dialysis to remove middle molecular weight toxin such as β2-MG, and try to avoid the use of calcium and warfarin and other drugs. If CVC causes the mitral stenosis, we need to control the heart rate and the symptoms of heart failure. If the disease is more severe, surgery is required, and the surgical options include endovascular balloon mitral valvuloplasty or valve replacement. In case of mitral regurgitation, drugs can be used including vasodilators and diuretics (or enhanced ultrafiltration). If CVC causes aortic regurgitation, we need to control blood pressure and heart rate. For severe aortic valve stenosis, heart valve replacement, or transcatheter aortic valve implantation (TAVI), surgery should be considered (6).
In previous studies (28,29), high calcium, high Pi, and high PTH in uremia patients were the risk factors for vascular calcification in uremia patients. In this study, calcium and Pi were risk factors for CVC, but in the multivariate analysis model, calcium, Pi, and PTH were no longer independent risk factors for CVC. This may be related to the relatively insufficient sample size in this study.
Every study has its limitations, and ours is no exception. Firstly, the echo examinations in this study were all performed at the same point in time, and some patients may have developed CVC during follow-up, implying that the true association between CVC and mortality is more robust than what we observed. And some of these patients had baseline CVC with CKD and we may be overestimating with our observation. Second, because this was a retrospective cohort study, the echo results were not read by a single person, which may have introduced bias. Third, we know that the dialysis membranes of different dialyzers have different clearance rates of β2-MG. This study only ensured that the same dialyzer was used within half a year of data collection, so different dialysis membranes have certain biases in the clearance rates of β2-MG. Finally, this was a single-center retrospective study with small sample size. Large-scale, long-term, and prospective studies are needed to confirm whether β2-MG levels can accurately predict the occurrence of CVC.
Conclusions
This study showed a disproportionately increased levels of β2-MG in CVC group, indicating that there is a certain relationship between β2-MG and CVC. We speculate that elevated β2-MG may promote the development of CVC. More prospective studies to look at temporal relationship between ESRD, levels of β2-MG, and incidence of CVC can establish this hypothesis and pave way for interventional studies.
Acknowledgments
We would like to express our gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided. We would like to express our gratitude to all those who offered their valuable help during the writing of this manuscript.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1185/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1185/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1185/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1185/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First People’s Hospital of Nantong City (No. 2019KW008) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moody WE, Edwards NC, Madhani M, et al. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: cause or association? Atherosclerosis 2012;223:86-94. [Crossref] [PubMed]
- Lu ML, Gupta S, Romero-Corral A, et al. Cardiac Calcifications on Echocardiography Are Associated with Mortality and Stroke. J Am Soc Echocardiogr 2016;29:1171-8. [Crossref] [PubMed]
- Bai J, Zhang X, Zhang A, et al. Cardiac valve calcification is associated with mortality in hemodialysis patients: a retrospective cohort study. BMC Nephrol 2022;23:43. [Crossref] [PubMed]
- Guan J, Xie H, Wang H, et al. Cardiac valve calcification as a predictor of cardiovascular outcomes in peritoneal dialysis patients: an inverse probability of treatment weighting analysis. Int Urol Nephrol 2023;55:1271-8. [Crossref] [PubMed]
- Samad Z, Sivak JA, Phelan M, et al. Prevalence and Outcomes of Left-Sided Valvular Heart Disease Associated With Chronic Kidney Disease. J Am Heart Assoc 2017;6:e006044. [Crossref] [PubMed]
- Ureña-Torres P, D'Marco L, Raggi P, et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant 2020;35:2046-53. [Crossref] [PubMed]
- Wang Z, Jiang A, Wei F, et al. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: a meta-analysis. BMC Cardiovasc Disord 2018;18:12. [Crossref] [PubMed]
- Mahon C, Davies A, Gambaro A, et al. Association of individual aortic leaflet calcification on paravalvular regurgitation and conduction abnormalities with self-expanding trans-catheter aortic valve insertion. Quant Imaging Med Surg 2021;11:1970-82. [Crossref] [PubMed]
- Yan T, Chopp M, Chen J. Experimental animal models and inflammatory cellular changes in cerebral ischemic and hemorrhagic stroke. Neurosci Bull 2015;31:717-34. [Crossref] [PubMed]
- You L, Xie R, Hu H, et al. High levels of serum β2-microglobulin predict severity of coronary artery disease. BMC Cardiovasc Disord 2017;17:71. [Crossref] [PubMed]
- Zhang J, Lu X, Zu Y, et al. Prognostic value of beta-2 microglobulin on mortality in chronic kidney disease patients: A systematic review and meta-analysis. Ther Apher Dial 2022;26:267-74. [Crossref] [PubMed]
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7. [Crossref] [PubMed]
- Ikee R, Honda K, Oka M, et al. Association of heart valve calcification with malnutrition-inflammation complex syndrome, beta-microglobulin, and carotid intima media thickness in patients on hemodialysis. Ther Apher Dial 2008;12:464-8. [Crossref] [PubMed]
- Lin FJ, Zhang X, Huang LS, et al. De novo Cardiac Valve Calcification after Hemodialysis in End-Stage Renal Disease Patients Predicts Future Cardiovascular Events: A Longitudinal Cohort Study. Cardiorenal Med 2019;9:229-39. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Kardiol Pol 2018;76:1-62. [Crossref] [PubMed]
- Liu DD, Zhang JC, Zhang Q, et al. TGF-β/BMP signaling pathway is involved in cerium-promoted osteogenic differentiation of mesenchymal stem cells. J Cell Biochem 2013;114:1105-14. [Crossref] [PubMed]
- Burotto M, Chiou VL, Lee JM, et al. The MAPK pathway across different malignancies: a new perspective. Cancer 2014;120:3446-56. [Crossref] [PubMed]
- Dayanand P, Sandhyavenu H, Dayanand S, et al. Role of Bisphosphonates in Vascular calcification and Bone Metabolism: A Clinical Summary. Curr Cardiol Rev 2018;14:192-9. [Crossref] [PubMed]
- Plytzanopoulou P, Papasotiriou M, Politis P, et al. Cardiac valve calcification in patients on maintenance dialysis. The role of malnutrition-inflammation syndrome, adiposity andcomponents of sarcopenia. A cross-sectional study. Clin Nutr ESPEN 2022;52:421-30. [Crossref] [PubMed]
- Berggård I, Bearn AG. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem 1968;243:4095-103.
- Xie J, Yi Q. Beta2-microglobulin as a potential initiator of inflammatory responses. Trends Immunol 2003;24:228-9; author reply 229-30. [Crossref] [PubMed]
- Carreras N, Arnaez J, Valls A, et al. CSF neopterin and beta-2-microglobulin as inflammation biomarkers in newborns with hypoxic-ischemic encephalopathy. Pediatr Res 2023;93:1328-35. [Crossref] [PubMed]
- Amighi J, Hoke M, Mlekusch W, et al. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke 2011;42:1826-33. [Crossref] [PubMed]
- Schillinger M, Exner M, Mlekusch W, et al. Inflammation and Carotid Artery--Risk for Atherosclerosis Study (ICARAS). Circulation 2005;111:2203-9. [Crossref] [PubMed]
- Vidula H, Tian L, Liu K, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med 2008;148:85-93. [Crossref] [PubMed]
- Jadoul M, Drüeke TB. β2 microglobulin amyloidosis: an update 30 years later. Nephrol Dial Transplant 2016;31:507-9. [Crossref] [PubMed]
- Ikee R, Honda K, Ishioka K, et al. Differences in associated factors between aortic and mitral valve calcification in hemodialysis. Hypertens Res 2010;33:622-6. [Crossref] [PubMed]
- Avila M, Mora C, Prado MDC, et al. Osteoprotegerin Is the Strongest Predictor for Progression of Arterial Calcification in Peritoneal Dialysis Patients. Am J Nephrol 2017;46:39-46. [Crossref] [PubMed]
- Yamada S, Oshima M, Watanabe Y, et al. Arterial location-specific calcification at the carotid artery and aortic arch for chronic kidney disease, diabetes mellitus, hypertension, and dyslipidemia. Calcif Tissue Int 2014;95:267-74. [Crossref] [PubMed]
(English Language Editor: J. Jones)


