
Real-world effectiveness of azvudine for patients infected with the SARS-CoV-2 omicron subvariant BA.5 in an intensive care unit
Highlight box
Key findings
• Patients administered azvudine showed a reduction in inflammatory indicators.
What is known, and what is new?
• A shorter intensive care unit stay was observed in younger compared to older patients.
• Azvudine usage led to a decrease in inflammatory markers.
What is the implication, and what should change now?
• A multi-center clinical study will be conducted to further evaluate azvudine’s real-world effectiveness. It is anticipated that azvudine can contribute positively to manage the coronavirus disease 2019 pandemic.
Introduction
Since the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in late 2019, thousands of mutations have surfaced, posing an unparalleled challenge to global public health. As of September 19, 2022, the World Health Organization had reported over 609 million confirmed cases and approximately 6.5 million fatalities worldwide. On August 1, 2022, an outbreak of the novel coronavirus disease 2019 (COVID-19) disease, attributed to the Omicron BA.5.1.3 variant, was noted in Sanya, Hainan Province, China (1). The Omicron variant, a mutant strain of the SARS-CoV-2 virus, exhibits increased transmissibility and a more pronounced latent period (2-4). Despite ongoing global vaccination efforts, a recent study revealed a significant decline in the humoral immune response to SARS-CoV-2 over time, implying a sustained risk of infection post-immunization (5). The continued global proliferation of the pandemic poses a substantial threat to public health, emphasizing an urgent need for antiviral therapeutics which can pose efficacy against all SARS-CoV-2 mutations.
Azvudine (FNC) is a synthetic nucleoside analog and represents a broad-spectrum of oral RNA small-molecule antiviral drug. It was originally developed in China for the treatment of human immunodeficiency virus type 1 (HIV-1) infected adult patients with a high viral load (6-8). FNC, once inside the host cell, undergoes phosphorylation by kinase to convert it into an active compound, the nucleoside triphosphate. This active form disrupts viral RNA synthesis during replication, thereby inhibiting the virus’s ability to reproduce. FNC also acts on the viral RNA-dependent RNA polymerase, terminating viral RNA synthesis during reverse transcription, which further impedes viral replication. After administration, FNC is primarily distributed to the thymus, where it undergoes triple phosphorylation and boosts the immune response (8). Recent studies have indicated the potential of FNC in managing COVID-19 (9,10). Its mechanism is based on nucleic acid negative conversion (NANC), particularly by shortening the time required for NANC in mild to moderate cases compared to standard antiviral therapy. Subsequently, on July 25, 2022, the National Medical Products Administration in China granted the approval for FNC to be applied to COVID-19 treatment, making it the first domestically produced oral antiviral drug to have gained approval in China. FNC further received recognition on August 9, 2022, with its inclusion in the “Treatment Protocol for Novel Coronavirus Pneumonia (9th Edition)” for treating typical adult COVID-19 patients by the State Health Care Commission and the State Administration of Traditional Chinese Medicine. Clinical application of FNC in the Sanya Hospital started on August 13, 2022, for treating COVID-19. Compared with standard antiviral therapy (including molnupiravir and paxlovid), FNC has demonstrated improved patient outcomes, notably in shortening the time to NANC and enhancing lung function in severe COVID-19 patients (11). Importantly, no adverse events have been associated with FNC treatment, further strengthening its promise as a COVID-19 therapeutic.
Current literature regarding the clinical characteristics of the Omicron BA.5 variant of SARS-CoV-2 is sparse. This study aimed to fill this gap by retrospectively analyzing the clinical characteristics of critically ill patients infected with Omicron BA.5 who were treated at intensive care unit (ICU). A further objective of this study was to evaluate the real-world effectiveness of FNC in treating such patients. FNC has shown promising antiviral activity against HCoV-OC43 and SARS-CoV-2 in preliminary in vitro studies (unpublished results). Results from the phase II clinical trial (GQ-FNC-201) of FNC for treating HIV infections demonstrated excellent safety and efficacy of this drug. Given that COVID-19 has been declared a public health emergency of international concern, it is imperative to expand our understanding of potential treatment options. This study represents an important real-world clinical trial investigating the efficacy and safety of FNC in treating COVID-19 patients. The goal is to enhance the existing body of data concerning FNC’s use, specifically within an ICU context, to provide practical insights for the management of critically ill COVID-19 patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1093/rc).
Methods
Patients
This study comprised of patients admitted to the Sanya Central Hospital’s ICU during the Sanya Omicron BA.5.1.3 variant outbreak from August 13, 2022 to September 7, 2022. Infections were confirmed via real-time reverse transcription-polymerase chain reaction (PCR) assays of nasopharyngeal swabs. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Sanya Central Hospital Ethics Committee approved the study (No. LLKY220877), and informed consent was waived due to its retrospective nature.
Data collection
Data were collected on patients’ general health status, pre-existing conditions, initial symptoms, lung infection status, and laboratory indicators. Disease severity was categorized as mild, moderate, severe, or critical according to the criteria laid out in the “Novel Coronavirus Pneumonia Treatment Protocol (Trial Version 9)” by the National Health and Wellness Commission of the People’s Republic of China. Key timeline events such as admission, ICU admission, ICU discharge, and hospital discharge were documented. The PCR analysis was performed utilizing the ABI7500 Analyzer. Each test included both positive and negative quality control samples for calibration purposes. Cycle threshold (Ct) values were monitored at regular intervals, including at ICU admission and discharge, with any changes duly noted. Additional data points included the time of the first negative PCR test, the interval between the first positive and negative tests, and the interval between two tests with a Ct value of ≥35 or between a negative and the first positive test, referred to as “time to NANC”. To assess the impact of FNC on ICU patients, subjects were divided into two treatment groups: the FNC group, which received FNC along with standard supportive therapy and traditional Chinese medicine (TCM), and the non-FNC group, which received only the standard supportive therapy and TCM (12). The duration for FNC administration lasted 5–9 days until the patient achieved NANC. All patients’ time to NANC was assessed.
Case confirmation and clinical staging were conducted following the criteria set out in the “Treatment Protocol for Novel Coronavirus Infection” (Trial Version 9), issued by the National Health Commission on February 4, 2020. The classification criteria included: (I) mild: patients exhibiting mild clinical symptoms and no imaging evidence of pneumonia. (II) Moderate: patients exhibiting symptoms of fever, respiratory issues, and imaging evidence of pneumonia. (III) Severe: patients meeting any of the following conditions: respiratory distress (respiratory rate ≥30 breaths/min), resting oxygen saturation ≤93%, or arterial partial pressure of oxygen (PaO2)/inhaled oxygen concentration (FiO2) ≤300 mmHg. (IV) Critical: patients exhibiting any of the following conditions: respiratory failure requiring mechanical ventilation, shock, or multi-organ failure requiring ICU treatment (13).
Patients were discharged or released from isolation based on the following criteria: (I) two consecutive PCR tests (at least 24 hours apart) indicating NANC (Ct ≥35). (II) Normal body temperature maintained for three days following the NANC tests. (III) Significant reduction in respiratory symptoms and substantial improvements in lung imaging, demonstrating lessened inflammation. Patients were then either discharged from isolation or transferred to a suitable unit for treatment of other conditions based on their health status.
Statistical analysis
Data were analyzed using SPSS 27.0 software. Normally distributed continuous variables were expressed as means ± standard deviation (SD), while non-normally distributed variables were reported as median [range or interquartile range (IQR)]. Categorical variables were expressed as n (%). Survival analysis and between-group comparisons were conducted using Kaplan-Meier curves.
Results
Baseline characteristics
This study included 13 ICU patients, with a male-to-female ratio of 9:4. The patient age distribution was as follows: five patients were aged less than 60 years, three were aged between 60–80 years, and five were aged over 80 years. Patients were also categorized into two severity groups: severe (including critical and severe patients; eleven patients in total) and non-severe (including moderate and mild patients; two patients). The FNC group consisted of six patients (three males and three females), ranging in age from 48 to 90 years, with a mean age of 72.33±15.86 years. It included three critical patients, two severe cases, and one moderate case. Initial nucleic acid Ct values ranged from 14.65 to 21.85, with a mean value of 17.22±1.38. The non-FNC group included seven patients (six males and one female), ranging in age from 42 to 89 years, with a mean age of 65.57±17.12 years. It comprised of three critical patients, three severe cases, and one mild case (Table 1).
Table 1
| Patient | Group | Age (years) | Sex | Severity of COVID-19 | Semi-quantitative assessment | Severity of inflammation | Symptoms | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| 1 | FNC | 57 | M | Moderate | 0 | No infection | Cough, white sputum, loose feces | Uremia, CKD stage 5, hypertension, secondary hyperparathyroidism, CRRT |
| 2 | FNC | 84 | M | Critical | 1/4 | Mild | Shortness of breath, gatism, disturbance of consciousness | Diabetes, hypertension, cerebral infarction, ECMO |
| 3 | FNC | 90 | F | Critical | 1/4 | Mild | Fever, fatigue | Gastric cancer, cerebral infarction, anemia, hypertension, coronary heart disease, diabetes, esophageal fissure hernia, duodenal diverticulum, abdominal hernia, chronic obstructive pulmonary disease, cerebral hemorrhage, surgical history of fracture |
| 4 | FNC | 68 | F | Critical | 1/4 | Mild | Chest tightness, shortness of breath, cough, expectoration | CKD stage 5, hypertension, hypertensive kidney disease, CRRT |
| 5 | FNC | 48 | M | Severe | 1/4 | Mild | Shortness of breath | Rheumatic heart disease |
| 6 | FNC | 87 | F | Severe | 1/4 | Mild | Cough, expectoration | None |
| 7 | Non-FNC | 84 | M | Critical | 1/4 | Mild | Fever | Surgical history of subdural hematoma removal + intracerebral hematoma removal + craniotomy decompression, CRRT |
| 8 | Non-FNC | 45 | M | Critical | 1/4 | Mild | Chest tightness, shortness of breath, breathing hard, fever | Pain in the left lower extremity, surgical history of fracture, CRRT |
| 9 | Non-FNC | 42 | M | Severe | 1/4 | Moderate | Abdominal pain, diarrhea | Esophageal and gastric varices bleeding, liver cancer with portal vein tumor thrombus, decompensated cirrhosis, chronic liver failure, hepatic encephalopathy |
| 10 | Non-FNC | 70 | M | Severe | 2/4 | Mild | Cough, expectoration, shortness of breath | Pulmonary tuberculosis, surgical histories of eyeball removal, esophagectomy and gastrectomy, ECMO, CRRT |
| 11 | Non-FNC | 89 | F | Critical | 1/4 | Mild | Shortness of breath | Hypertension, coronary heart disease, cerebral infarction |
| 12 | Non-FNC | 59 | M | Severe | NA | NA | Rash | Diabetes, hypertension, cerebral infarction |
| 13 | Non-FNC | 67 | M | Mild | 1/4 | Mild | Fever, cough | Hypertension, cerebral infarction |
COVID-19, coronavirus disease 2019; FNC, azvudine; M, male; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; F, female; NA, not applicable.
Underlying diseases
Patients across both groups exhibited varying degrees of underlying health conditions. The six patients in the FNC group had a total of 11 underlying diseases, including five patients with heart conditions such as hypertension, coronary artery disease, rheumatic heart disease, and heart failure. Two patients suffered from pulmonary disease, both presenting respiratory failure, one with chronic obstructive pneumonia and the other with severe pneumonia. Two patients had renal insufficiency, both with renal hypertension, anemia, and requiring hemodialysis. Two patients had brain conditions: cerebral hemorrhage and cerebral infarction. Three had metabolic diseases, including two cases of diabetes and one of secondary parathyroidism. One patient was diagnosed with gastric cancer, and one had no underlying conditions.
The non-FNC group consisted of seven patients with a total of 11 underlying diseases. Three patients had cardiovascular disease, including cerebral infarction, coronary heart disease, and hypertension. Two patients had cancers, one with esophageal cancer and the other with hepatocellular carcinoma, the latter presenting severe complications such as lung infection, pleural effusion, peritoneal effusion, and liver failure. The most common underlying diseases among all patients were hypertension, cerebral infarction, type 2 diabetes mellitus, and chronic renal insufficiency (Table 1).
Clinical features
All the 13 patients in this study presented with clinical symptoms upon admission, with a range of six distinct symptoms observed in both FNC group and non-FNC groups. In the FNC group, three patients experienced coughing and sputum production, and two patients presented with shortness of breath and wheezing. Fever was reported by one patient, and gastrointestinal symptoms were observed in another patient. Half of the patients in the non-FNC group exhibited fever, cough, and shortness of breath, two patients reported gastrointestinal discomfort, including abdominal pain and diarrhea, and one patient presented with urinary symptoms explicit in frequency and urgency. The three most common clinical symptoms across all patients were shortness of breath, fever, and cough with sputum.
Imaging features
Lung imaging was performed using a 64-layer spiral CT scan. In the FNC group, six patients underwent this examination, with five patients displaying bilateral inflammation and no significant imaging abnormalities noted in the remaining patients. Six patients in the non-FNC group underwent the same CT examination, all showed signs of inflammatory lung changes. Interestingly, one patient in this group displayed mild imaging changes upon initial admission but developed multiple patchy, slightly dense shadows in both lungs on the sixth day of admission.
The severity of the inflammation, ranging from mild to critical, was classified based on semi-quantitative assessments of the lung images. Herein, an inflammation occupying 1/4 of the lung images was labeled as mild, 2/4 as moderate, and any inflammation extending beyond 3/4 of the lung images was considered (critically) severe. In the FNC group, five cases were categorized as mild and one as no inflammation. The non-FNC group showed five mild and one moderate cases. No statistical significance was found between the two groups (P>0.05).
Laboratory tests
Existing studies suggest that in the early stages of COVID-19, patients typically present with normal or diminished total leukocyte counts in peripheral blood (14-18). Some patients exhibit lymphopenia, while others may demonstrate elevated liver enzymes, lactate dehydrogenase, muscle enzymes, myoglobin, and troponin. Most patients show an increase in C-reactive protein (CRP), with procalcitonin (PCT) levels remaining within the normal range. Severe and critical patients may exhibit elevated D-dimer, a consistent decrease in peripheral blood lymphocytes, and a progressive increase in inflammatory markers, such as interleukin-6 (IL-6) and CRP.
In the non-FNC group, data for IL-6 and serum troponin levels were unavailable for one patient. Nonetheless, this patient exhibited significantly elevated ultra-sensitive CRP and procalcitonin levels during the same period. There were no statistically significant differences in total leukocytes, absolute neutrophil values, absolute lymphocyte values, IL-6, glutamate aminotransferase, creatine kinase, serum troponin, PCT, or the number of changes between the FNC and non-FNC groups (P>0.05). Conversely, patients in the FNC group demonstrated lower inflammatory markers and D-dimers after 5-day treatment. Detailed laboratory results are presented in Table 2.
Table 2
| Variables | FNC group (n=6) | Non-FNC group (n=7) | P value |
|---|---|---|---|
| WBC (×109/L) | 5.89±3.79 (3.60–5.76) | 8.54±4.53 (5.49–9.85) | 0.76 |
| NEUT (×109/L) | 4.26±4.03 (1.66–4.29) | 7.06±4.58 (3.54–8.73) | 0.49 |
| LYMPH (×109/L) | 1.13±0.39 (0.84–1.28) | 0.67±0.37 (0.49–0.83) | 0.22 |
| hs-CRP (mg/L) | 29.91±34.83 (5.49–36.07) | 80.38±64.62 (15.73–135.85) | <0.05 |
| IL-6 (pg/mL) | 215.36±212.36 (78.34–213.76) | 141.96±125.14 (67.62–149.33) | 0.52 |
| ALT (U/L) | 18.33±18.93 (6.5–18.25) | 36.86±47.08 (9.5–34.5) | <0.05 |
| AST (U/L) | 36.83±30.89 (18.75–34.5) | 124.43±191.06 (21.5–93) | <0.05 |
| LDH (U/L) | 257.67±68.57 (213.75–262.25) | 418.83±363.27 (218.5–336.75) | <0.05 |
| CPK (U/L) | 111.67±67.02 (66–144.25) | 367.83±535.89 (92.5–204.5) | <0.05 |
| CTNI (ng/mL) | 0.03±0.02 (0.03–0.04) | 0.03±0.03 (0.01–0.03) | 0.71 |
| PCT (ng/mL) | 0.59±0.49 (0.18–0.93) | 7.15±13.34 (0.14–5.08) | 0.12 |
| D-dimer (mg/mL) | 20.54±41.86 (1.26–3.49) | 3.32±4.15 (0.88–3.05) | <0.05 |
The data are presented as mean ± standard deviation (range). FNC, azvudine; WBC, white blood cell; NEUT, neutrophils; LYMPH, lymphocytes; hs-CRP, high-sensitivity c-reactive protein; IL-6, interleukin-6; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; CTNI, cardiac troponin I; PCT, procalcitonin.
Detailed treatment modalities for both groups of patients
Two patients in the FNC group and three patients in the non-FNC group received continuous renal replacement therapy (CRRT). Extracorporeal membrane oxygenation (ECMO) was administered in one case in each group. The Lotus Qing Fei capsule (a TCM) was given to two patients in the FNC group and three patients in the non-FNC group, and sivelestat sodium for injection was administered in one case per group (P>0.05). In the non-FNC group, hydrocortisone was used at a dose of 200 mg daily for one day. Antibacterial therapy was provided to three patients in the FNC group, including one case treated with seven different antibiotics, one case treated with two types of antibiotics, and one case receiving a regimen of three types of antibiotics. In the non-FNC group, antibacterial therapy was provided to seven patients: one case involved treatment with seven different antibiotics, another case with three types of antibiotics, and the remaining cases each utilized a single type of antibiotic for treatment.
Immunomodulatory agents used included thymalfasin thyroxine for injection (1.6 mg/stem), COVID-19 human immunoglobulin (5,000 U/bottle, 1.25 g/25 mL), and human immunoglobulin (2.5 g/bottle). Immunomodulators were administered to five patients in each group, with no statistically significant difference between the two groups (P>0.05). In addition, both the FNC and non-FNC groups received Tongkat treatment with dialectical evidence guided by TCM theory.
The administration of FNC, dosed at 5 mg per day orally or via nasal feeding, was initiated in each patient. Of these, three patients completed the course through oral or nasal feeding, while the remaining three transitioned from oral to nasal feeding. The specifics of the dosing regimen can be seen in Table 3. Thymofacine was administered at a dose of 1.6 mg every 12 hours subcutaneously. The duration of treatment was significantly longer in the FNC group (16.00±3.46 days) compared to the non-FNC group (5.50±0.70 days), excluding the possible influence of the duration of thymofacine treatment on the time to NANC (P<0.05).
Table 3
| Variables | FNC group (n=6), n (%) |
Non-FNC group (n=7), n (%) |
|---|---|---|
| Lianhua Qingwen Granule | 2 (33.33) | 3 (42.86) |
| Sivelestat sodium hydrate | 1 (16.67) | 1 (14.29) |
| Antibacterial | 3 (50.00) | 7 (100.00) |
| Immunopotentiator | 5 (83.33) | 5 (71.43) |
| TCM decoction medicine | 6 (100.00) | 7 (100.00) |
| CRRT | 2 (33.33) | 3 (42.86) |
| ECMO | 1 (16.67) | 1 (14.29) |
| Antacid | 4 (66.67) | 1 (14.29) |
FNC, azvudine; TCM, traditional Chinese medicine; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
In the FNC group, the average duration of FNC administration was 7.71±1.60 days. Subgroups A1 and A2 were formed based on the interval between the first positive SARS-CoV-2 test and the initiation of FNC, with A1 receiving FNC within 5 days and A2 after 5 days. The average time to NANC was 13.60±5.50 days for subgroup A1 and 17.5±2.12 days for subgroup A2 (P>0.05). The time taken to reach NANC in the FNC group ranged from 6 to 21 days, with a median of 16 days and an average time of 14.11±5.74 days. In contrast, the non-FNC group had a wider range of 7 to 30 days, with a median of 13.5 days and an average time to NANC of 16.33±6.67 days. An examination of nucleic acid regression, safety, and results, as presented in Figure 1 and Table 4, indicated no complications related to the use of FNC.
Table 4
| Patient | Group | Nucleic acid negative conversion, days | Days of conversion to negative after azvudine treatment | Length of ICU stay, days |
Nucleic acid Ct value | Outcome (recovery, discharge, death, etc.) |
|---|---|---|---|---|---|---|
| 1 | FNC | 16 | 6 | 4 | 40/40 | Discharged |
| 2 | FNC | 13 | 12 | 13 | 40/40 | Cured of COVID-19 and died after discharge |
| 3 | FNC | 15 | 11 | 14 | 40/40 | Discharged |
| 4 | FNC | 12 | 10 | 12 | 40/40 | Discharged |
| 5 | FNC | 6 | 4 | 6 | 40/40 | Discharged |
| 6 | FNC | 21 | / | 4 | 40/40 | Discharged |
| 7 | Non-FNC | 19 | 13 | 4 | 40/40 | Discharged |
| 8 | Non-FNC | 14 | / | 14 | 40/40 | Discharged |
| 9 | Non-FNC | 13 | / | 14 | 40/40 | Discharged |
| 10 | Non-FNC | 11 | / | 9 | 40/40 | Discharged |
| 11 | Non-FNC | 14 | / | 16 | 40/40 | Discharged |
| 12 | Non-FNC | 15 | / | 5 | 40/40 | Discharged |
| 13 | Non-FNC | 31 | / | 17 | 40/40 | Discharged |
ICU, intensive care unit; Ct, cycle threshold; FNC, azvudine; COVID-19, coronavirus disease 2019.
Analysis of results based on different endpoints
In interpreting our results, due to the different baseline characteristics of patients in the two groups and variations in gender, age, underlying disease, and disease severity between the FNC group and the non-FNC group, we considered different endpoint events to differentiate between the two groups.
Differences between groups of time from the first positive nucleic acid test to the first negative nucleic acid test of patients
The interval from the first positive to the first negative nucleic acid test served as the endpoint event. The categorizations of disease severity, patient age, patient gender, and patient comorbidities formed the basis for grouping, in order to assess differences between groups who received FNC and those who did not. As illustrated in Figure 2A, no significant differences were observed in the P values for the FNC and non-FNC groups with different disease severity, standing at 0.39 and 0.99, respectively (P>0.05). Figure 2B demonstrates that there were no statistically significant differences (P>0.05) in the P values of 0.81, 0.63, and 0.58 for FNC and non-FNC users aged <60, 60–80, and >80 years, respectively.
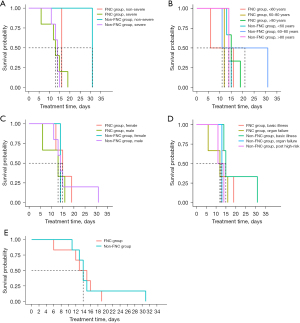
Gender-based analysis, as shown in Figure 2C, revealed P values of 0.27 (P>0.05) for those administered FNC and those without, respectively, signifying no significant differences. In terms of patients with underlying diseases, Figure 2D exhibits the P values for the FNC and non-FNC groups as 0.84, 0.19, and 0.14 (P>0.05), indicating no significant differences for patients with high intraoperative risk, organ failure, and other comorbidities, respectively. When we considered the interval from the first positive to the first negative test for the 13 ICU patients who were part of the FNC and non-FNC groups, there were no significant differences even without considering groupings, as portrayed in Figure 2E.
Differences between groups of time from treatment initiation to the first negative nucleic acid test
Given the potential variations in the duration of medication administration across patients in the FNC group and non-FNC group, the duration of hospital stay and the length of medication treatment were respectively selected as endpoints. The eventual results were visible solely in the distinct disease severity subgroups of patients suffering from COVID-19, as depicted in Figure 3A. Notably, a decrease was observed in the duration among the severe group, with P values of 0.98 and 0.99, respectively (P>0.05). However, no statistically significant differences were apparent. The remaining groups were divided based on age, gender, and comorbidities to assess differences between those who did and did not receive FNC. As presented in Figure 3B-3D, no substantial differences were discerned in survival curves, irrespective of the disease severity, patient age, gender, or comorbidities. Consequently, for these 13 ICU patients, the administration of FNC did not alter the time from hospitalization to the first NANC.
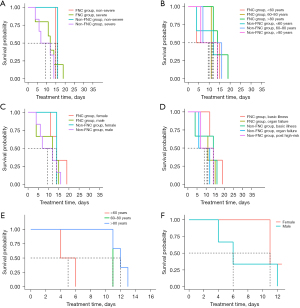
When only considering the six patients receiving FNC as the study population, as shown in Figures 3E,3F, younger patients (those aged <60 years) experienced a shorter NANC time in comparison to their counterparts aged 60–80 years and those aged >80 years. Similarly, male patients had a shorter NANC time when compared to females. However, due to the small sample size, a degree of bias was observed, with a P value of 0.36 (P>0.05), and no statistically significant difference was detected. Therefore, for these six ICU patients receiving FNC, none of the four baseline variables (severity of COVID-19, age, sex, and comorbidities) were deemed significant. While these differences did not reach statistical significance, likely because of our small sample size, there was a noticeable numerical trend favoring patients aged under 60 years. This might be influenced by the tendency for younger male patients to contract the disease, suggesting potential clinical benefits.
Upon revisiting the potential discrepancy in medication duration between patients in the FNC and non-FNC groups and again selecting the length of hospital stay and medication duration as endpoints, the results were restricted to the different disease severity of patients with COVID-19. Although a slight reduction in the duration was noted for the severe group with P values of 0.16 and 0.26, respectively (P>0.05), there were still no significant differences. The rest of the groups, categorized according to age, sex, and comorbidities and evaluated on the differences between those who did and did not receive FNC, showed no significant differences in survival curves based on disease severity, patient age, sex, or comorbidities. Therefore, for the 13 ICU patients, FNC use did not impact the time from hospitalization to the first NANC. Notably, focusing solely on the six patients on FNC, younger male patients had a faster recovery time. However, due to the small sample size, there was a degree of bias, with a P value of 0.21 (P>0.05), and no significant difference was found. For these six ICU patients on FNC, none of the four baseline variables proved significant, though there was a numerical advantage for patients aged under 60 years.
Differences between groups in the duration of ICU stay
The variations between groups that either did or did not receive FNC were evaluated separately, employing the duration of patients’ stay in the ICU as the endpoint. Groupings were based on the disease severity of patients with COVID-19, patient age, patient gender, and comorbidities, as depicted in Figures 4A,4B. Among the 13 patients in this cohort, there appeared to be a reduction in the duration of ICU stay, though no significant differences were evident between the FNC and non-FNC groups.

When the initiation of medication was taken as the starting point to calculate the total time spent outside the ICU, it was revealed that, for these 13 ICU patients, the administration of FNC did influence the duration of ICU stay, with a noticeable reduction in the overall time for younger patients in comparison to older patients. Upon further individual analysis of the six patients who received FNC, as represented in Figure 4C,4D, it was found that none of the four baseline variables significantly influenced the time from the commencement of FNC to ICU discharge. However, a shorter duration was observed in the subgroup of patients aged under 60 years. Additionally, the subgroup of male patients suggested a possible higher prevalence of the disease in younger males.
Revisiting the differences between groups that did or did not receive FNC, evaluated separately using the duration of ICU stay as the endpoint and maintaining the grouping based on disease severity, patient age, patient gender, and comorbidities; it was reiterated that in this cohort of 13 ICU patients, the duration of ICU stay was reduced. However, no significant differences were apparent when comparing the FNC group to the non-FNC group. Upon considering the initiation of medication as the starting point for the total time spent outside the ICU, it emerged that FNC use did not significantly affect the duration of ICU stay among these 13 ICU patients. However, a reduction was observed in the overall time for younger patients compared to older ones. Further analysis of the six patients who were administered FNC showed that none of the four baseline variables (severity of COVID-19, age, sex, and comorbidities) had a significant impact on the time from FNC initiation to ICU discharge, but the time was notably shorter for the patient group aged under 60 years.
Discussion
Nucleoside antiviral drugs, synthetic and chemically modified nucleoside analogs, mimic natural nucleosides to gain entry into host cells. Once inside, these analogs transform into an active compound, nucleoside triphosphate, via the catalysis of kinase. This active compound embeds itself in viral DNA or RNA during the viral DNA or RNA synthesis process, consequently terminating the viral DNA or RNA chain synthesis and inhibiting viral replication. Furthermore, nucleoside antiviral drugs can suppress the activity of virus DNA-dependent DNA polymerases (DdDps), RNA-dependent DNA polymerases (RdDps), and RNA-dependent RNA polymerases (RdRps), resulting in the inhibition of viral replication (11).
This preliminary study serves as a real-world clinical trial designed to assess the effectiveness and safety of FNC in treating COVID-19. The study focused on 13 COVID-19 patients characterized by severe disease risk factors. FNC, developed by a Chinese company, is a broad-spectrum oral RNA small-molecule antiviral drug (8). A small randomized clinical trial has reported that the 4-day NANC rate of FNC is 100% (10). However, this study was conducted at the ICU of a single center and had a low enrollment number. The omicron variant of SARS-CoV-2 typically causes less severe disease than other variants, yet patients can exhibit more severe symptoms due to underlying diseases (2-4,19). Hence, this study provides valuable insights for treating critically ill patients.
It is worth noting that FNC was administered intranasally, which diverged from the instruction of taking the tablets on an empty stomach, thus potentially affecting its full effectiveness. Simultaneous administration of other medications for comorbidities could result in drug interactions that might impact the efficacy of FNC (20-23). The specific pharmacological and toxicological effects of FNC in treating patients with the BA.5.1.3 variant require further clarification. Additionally, future studies with larger sample sizes are necessary. This study reports on 13 COVID-19 patients with severe disease risk factors. One of the significant findings from this study could be the absence of any adverse events related to the treatment, a fact that warrants further evaluation.
This study, marking the first investigation into the clinical characteristics of the omicron BA.5 variant, revealed no statistically significant differences (P>0.05) in general conditions, underlying diseases, clinical symptoms, and initial inflammatory extent in lung imaging between patients with or without FNC administration. Nevertheless, notable reductions in inflammatory indicators were observed in laboratory tests among the patient cohort receiving FNC. Furthermore, no significant difference was found in the time to NANC for the special population in the ICU treated with FNC.
Our study, while offering preliminary insights into the potential benefits of FNC against the Omicron BA.5.1.3 subvariant, is not without its limitations. Foremost, the small sample size of 13 patients restricted the generalizability of our findings to a broader population, and results should be interpreted with caution. The absence of a true control group poses another limitation. The diversity in treatments challenged the robustness of our comparative analysis, as there was no consistent standard of care against which FNC’s effects could be directly measured. Furthermore, the retrospective nature of our study inherently carried potential biases, such as selection bias and data incompleteness. While our findings provided an initial indication of FNC’s potential utility, larger, prospective, randomized controlled trials are essential to validate and build upon these observations.
Conclusions
These findings imply that FNC may be a feasible treatment option for the novel coronavirus omicron BA.5.1.3 variant, with no significant side effects. Moreover, FNC did not impact the time to NANC in the special population infected with SARS-CoV-2 omicron subvariant in the ICU and exhibited a commendable safety profile. It is anticipated that a national multi-center clinical study will be conducted to further determine the real-world effectiveness of FNC, which could potentially confer greater benefits to younger patients. Although these results offer a glimpse of hope to COVID-19 patients, further research remains imperative.
Acknowledgments
We express our gratitude to Dr. George Rakovich (University of Montreal School of Medicine, Canada) and Dr. Prateek Lohia (Wayne State University, USA) for their critical comments and valuable advice on this study.
Funding: This work was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1093/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1093/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1093/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1093/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Sanya Central Hospital Ethics Committee approved the study (No. LLKY220877), and informed consent was waived due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang X, Zhu X, Lin Y, et al. Tracking the first SARS-CoV-2 Omicron BA.5.1.3 outbreak in China. Front Microbiol 2023;14:1183633. [Crossref] [PubMed]
- Pather S, Madhi SA, Cowling BJ, et al. SARS-CoV-2 Omicron variants: burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines. Front Immunol 2023;14:1130539. [Crossref] [PubMed]
- Relan P, Motaze NV, Kothari K, et al. Severity and outcomes of Omicron variant of SARS-CoV-2 compared to Delta variant and severity of Omicron sublineages: a systematic review and metanalysis. BMJ Glob Health 2023;8:e012328. [Crossref] [PubMed]
- Silva SJRD, Kohl A, Pena L, et al. Recent insights into SARS-CoV-2 omicron variant. Rev Med Virol 2023;33:e2373. [Crossref] [PubMed]
- Chenchula S, Karunakaran P, Sharma S, et al. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J Med Virol 2022;94:2969-76. [Crossref] [PubMed]
- Yu B, Chang J. The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation (Camb) 2022;3:100321. [Crossref] [PubMed]
- Wang J, Xu W, Jin P. The first independently developed drug for the treatment of novel coronavirus pneumonia in China-Azovudine. Chinese Journal of Pharmacy 2022;57:2041-4.
- Wang RR, Yang QH, Luo RH, et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One 2014;9:e105617. [Crossref] [PubMed]
- Yu B, Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct Target Ther 2020;5:236. [Crossref] [PubMed]
- Ren Z, Luo H, Yu Z, et al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinh) 2020;7:e2001435. [Crossref] [PubMed]
- Zhong L, Zhao Z, Peng X, et al. Recent advances in small-molecular therapeutics for COVID-19. Precis Clin Med 2022;5:pbac024. [Crossref] [PubMed]
- Huang Y, Feng D, Li S, et al. Clinical characteristics of 95 cases of severe novel coronavirus pneumonia. Journal of Tropical Medicine 2022;22:1258-65.
- Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med 2022;28:1933-43. [Crossref] [PubMed]
- Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55:102763. [Crossref] [PubMed]
- Rahi MS, Jindal V, Reyes SP, et al. Hematologic disorders associated with COVID-19: a review. Ann Hematol 2021;100:309-20. [Crossref] [PubMed]
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020;95:834-47. [Crossref] [PubMed]
- Tavakolpour S, Rakhshandehroo T, Wei EX, et al. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett 2020;225:31-2. [Crossref] [PubMed]
- Illg Z, Muller G, Mueller M, et al. Analysis of absolute lymphocyte count in patients with COVID-19. Am J Emerg Med 2021;46:16-9. [Crossref] [PubMed]
- Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022;185:447-456.e11. [Crossref] [PubMed]
- Zhang X, Jiao F, Li G, et al. Elevated INR in a COVID-19 patient after concomitant administration of azvudine and anticoagulants. Front Pharmacol 2023;14:1191608. [Crossref] [PubMed]
- Yang H, Wang Z, Jiang C, et al. Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: Results of a real-world study. J Med Virol 2023;95:e28947. [Crossref] [PubMed]
- Zeng G, Wang L, Li J, et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19. J Med Virol 2023;95:e28836. [Crossref] [PubMed]
- Deng G, Li D, Sun Y, et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J Med Virol 2023;95:e28756. [Crossref] [PubMed]



