
Complications related to extracorporeal life support in lung transplantation: single-center analysis※
Highlight box
Key findings
• In a high-volume lung transplantation (LTx) center with an off-pump intra-operative extracorporeal life support (ECLS) strategy, ECLS is used in 22% of LTx cases of which 67% develop an ECLS-related complication.
What is known and what is new?
• ECLS is an essential part of every LTx program, however, the local practices of its use differ substantially among various large volume LTx centers.
• We provide a meticulous description of ECLS-related complications and report that not all LTx indications require the same need for intra-operative ECLS.
What is the implication, and what should change now?
• Since the majority of LTx procedures can be performed without ECLS, and ECLS comes with an unavoidable complication risk, its use should be carefully considered case-by-case. Further prospective studies are needed to achieve an international consensus on the use of ECLS during LTx.
Introduction
Lung transplantation (LTx) remains the only therapeutic option for patients with end-stage lung disease. Although the overall survival after LTx has improved over the years, the surgical procedure and post-LTx patient care remain challenging (1). Extracorporeal life support (ECLS) is often used to overcome the challenges like acute pre-transplant recipient deterioration, primary graft dysfunction (PGD) or other intra- and post-procedural events (2,3). Although encouraging results have been reported for patients bridged with ECLS to LTx (4-7) or for extended post-procedural ECLS of pulmonary arterial hypertension (PAH) patients (8-10), the routine use of intra-operative ECLS remains a matter of debate. In the past years the superiority of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) over cardiopulmonary bypass (CPB) has been shown (11-13). Intra-operative ECLS use ranges from 27–100% in different large-volume centers, depending on the choice of strategy between ECLS use-on-indication (“off-pump”) or routine use (14-16). Although a recent consensus document from The American Association of Thoracic Surgery provides recommendations about intra-operative ECLS use, there is still no international consensus in favor of either off-pump or routine ECLS strategy (17). ECLS is an essential part of every LTx program, however, its invasive nature might be a source of complications, as previously reported (14,15).
The aim of this study is to retrospectively analyze our experience with ECLS within our LTx program where an off-pump LTx strategy is used, as well as to describe the incidence and nature of ECLS-related complications. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-443/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). There is no experiment in this paper as it is a retrospective study of clinical strategies, therefore informed consent is not required. The study was approved by the local ethics committee (No. S64384/S51577).
Study design and patient selection
A retrospective single-center descriptive cohort study of patients undergoing LTx with ECLS at University Hospitals Leuven, Belgium was performed. All patients undergoing LTx in our center between January 1st, 2010 until December 31st, 2020 were assessed (n=728; Figure 1). Patients undergoing heart-LTx (n=8) or multi-organ transplantation (n=17) were excluded. Within the remaining cohort (n=703), those undergoing LTx with ECLS at any peri-operative timepoint were identified and included in the final analysis (n=156). To avoid patient selection bias, a descriptive analysis of the entire ECLS cohort was performed. In the off-pump cohort (n=547), only survival was assessed. Patients were followed-up until September 15th, 2022. Data were collected from electronic patient files.

ECLS strategy
At our center the standard procedure is to perform off-pump sequential single-LTx (SSLTx) through bilateral anterior thoracotomy. ECLS is used to anticipate and overcome hemodynamic and/or oxygenation problems occurring pre-, intra- or post-operatively. At the multidisciplinary pre-transplant meeting an ECLS strategy for each patient is discussed. Hilar test clamping is performed intra-operatively to assess need for ECLS and to avoid ECLS initiation in an acute setting. A decision to start ECLS is based on a case-by-case clinical assessment of hemodynamic and oxygenation parameters and gas exchange. Cannulation and initiation of ECLS is routinely performed by a member of the cardiac surgical team. In case of veno-venous (VV) or VA-ECMO, 100 IU/kg of unfractionated heparin is administered. CPB is reserved for major bleeding complications and concomitant cardiac procedures, increasing the heparin dose to 300–400 IU/kg.
ECLS cohort
Demographic data of donors and recipients were reviewed, as well as recipient variables that could influence the use of ECLS: transplant indication, high urgency status, previous thoracic surgery, pre-operative intensive care unit (ICU)-stay and mechanical ventilation. Reasons for ECLS initiation as well as ECLS characteristics including type, cannulation strategy and timing were analyzed and divided into groups with patients receiving ECLS as a bridge to LTx, intra- or post-operative support. In order to avoid listing of the same patient in several groups, the moment of ECLS initiation determined the group. If a patient received more than one type of ECLS support, only the most invasive one was considered (from least to most invasive: VV-ECMO < VA-ECMO < CPB).
Reflecting the post-procedural coagulation capacity, we analyzed international normalized ratio and thrombocytes level at admission to ICU and fluid balance. Regarding the short- and long-term outcomes in our ECLS cohort, we analyzed PGD grades (PGD-3) and incidence, length of ICU and hospital stay, 30- and 90-day mortality, incidence of chronic lung allograft dysfunction (CLAD), and 1-/5-year patient survival. PGD was graded according to the 2016 International Society for Heart and Lung Transplantation (ISHLT) consensus definition (18). CLAD was also determined following ISHLT definition (19). Survival was compared between patients who did and did not develop an ECLS-related complication and between patients undergoing LTx with and without ECLS. According to Leuven LTx program policy, all patients are followed-up in our center, therefore no patients were lost to follow-up.
To clarify the context of our findings, we compared our series with three large volume centers that recently published their ECLS experience in LTx (8,14,20).
ECLS-related complications
ECLS-related complications were defined prior to data collection and divided according to their direct or indirect relation to ECLS (21). As summarized and defined in Table 1, directly related are vascular complications (22-24), wound complications (25-27) and mechanical complications related to ECLS circuit and components (28,29). Indirectly related are bleeding and thromboembolic events (26,30), neurological complications (31,32) and presence of acute kidney injury (33,34).
Table 1
| Complication | Definition |
|---|---|
| Direct relation to ECLS | |
| Vascular | Vessel perforation, pseudoaneurysm, dissection, air embolism, limb compartment syndrome, limb ischemia |
| Wound cannulation site | Wound complications associated with cannulation and requiring VAC therapy or surgical intervention |
| Mechanical | Clots in the circuit, oxygenator failure, cannula thrombus |
| Indirect relation to ECLS | Definition |
| Hemothorax | Need for revision |
| Thromboembolism | Systemic thromboembolism, deep venous thrombosis |
| Neurological | Ischemic or bleeding CVA |
| Acute kidney injury | Decrease of renal function requiring CRRT |
ECLS, extracorporeal life support; VAC, vacuum-assisted closure therapy; CVA, cerebrovascular accident; CRRT, continuous renal replacement therapy.
Statistics
Continuous variables were expressed as median [interquartile range (IQR)], categorical variables as absolute numbers and frequencies (%). GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Kaplan-Meier analysis was used to assess patient survival (Log-rank test). Fisher’s exact text was used for comparison of demographic data between our series and other studies. P<0.05 was considered significant. Missing data were reported, no statistical analysis was performed.
Results
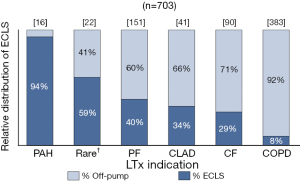
One hundred and fifty-six (22%) patients transplanted between 2010–2020 required intra-operative ECLS. The LTx indications with the highest incidence of ECLS were PAH, rare diseases and pulmonary fibrosis (PF) (Figure 2).
Pre- and post-transplant donor and recipient characteristics are summarized in Table 2. Lungs were procured following donation after brain-death in 124/156 (79%) cases. Median donor age was 49 years and 65/156 (42%) were female. Regarding recipients, median age was 52 years and 78/156 (50%) were female. One third of ECLS patients had a history of thoracic surgery, mostly performed through open thoracotomy. The median pre-operative systolic pulmonary artery pressure for patients measured with ultrasound (n=92) was 54 mmHg and for patients measured with right heart catheterization (n=64) mean pulmonary artery pressure was 32.5 mmHg. Twenty-six percent of ECLS-patients were hospitalized in ICU pre-LTx.
Table 2
| Characteristics | Values (N=156) |
|---|---|
| Donor characteristics | |
| National donor | 107 [69] |
| International donor (Eurotransplant) | 46 [29] |
| International donor (non-Eurotransplant) | 3 [2] |
| DBD | 124 [79] |
| DCD | 32 [21] |
| Female | 65 [42] |
| Age (years) | 49 [40–58] |
| Weight (kg) | 75 [66–83] |
| Height (cm) | 175 [165–180] |
| BMI (kg/m2) | 24.5 [22.8–26.3] |
| Smoking history | 55 [35] |
| Length of ventilation (hours) | 62 [38–120] |
| CMV mismatch (D+, R−) | 37 [24] |
| Pre-operative recipient characteristics | |
| Female | 78 [50] |
| Age (years) | 52 [36–59] |
| Weight (kg) | 64 [53–78.25] |
| Height (cm) | 169 [160–176] |
| BMI (kg/m2) | 22.1 [19.3–26.3] |
| BSA (m2) | 1.72 [1.58–1.93] |
| Indication to LTx | |
| Pulmonary fibrosis | 60 [38] |
| COPD | 29 [19] |
| Cystic fibrosis | 26 [17] |
| Pulmonary arterial hypertension | 15 [10] |
| Other disorders | 12 [8] |
| CLAD-BOS | 12 [8] |
| CLAD-RAS | 2 [1] |
| Previous surgery | 53 [34] |
| Thoracic | 48 [31] |
| Cardiac | 5 [3] |
| Surgical access (previous surgery) | |
| Open thoracotomy | 18 [12] |
| Video-assisted thoracoscopy | 17 [11] |
| Sternotomy | 15 [10] |
| Endovascular cardiac procedure | 2 [1] |
| Pre-operatively measured PAP | |
| US measured | 92 [59] |
| Systolic PAP (mmHg) | 54 [39.3–83.5] |
| RHC measured | 64 [41] |
| Systolic PAP (mmHg) | 52 [38–76] |
| Diastolic PAP (mmHg) | 22 [15–35] |
| Mean PAP (mmHg) | 32.5 [24–46.25] |
| Missing values | 46 [29] |
| Pre-operative status | |
| High urgency status | 33 [21] |
| Pre-operative ICU stay | 40 [26] |
| Pre-operative ICU stay (days) | 10 [6.75–16.25] |
| Pre-operative mechanical ventilation | 22 [14] |
| Pre-operative mechanical ventilation (days) | 8 [5.5–15.5] |
| Post-operative parameters & short-term outcome | |
| Any PGD grade 3 during 72 hours† | 72 [46] |
| PGD grade 3 at 72 hours | 35 [22] |
| INR at ICU admission | 1 [1–1.1] |
| Thrombocytes at ICU admission (109 cells/L) | 51.4 [35.25–74.75] |
| Positive total fluid balance in first 72 hours | 124 [79] |
| Negative total fluid balance in first 72 hours | 32 [21] |
| Length of ICU stay (days) | 10.5 [6–27] |
| Length of hospital stay (days) | 36 [25–57] |
| 30-day mortality | 9 [6] |
| 90-day mortality‡ | 13 [8] |
| Long-term outcome | |
| CLAD incidence | 37 [24] |
| CLAD-free survival (days) | 919 [512–2,159] |
| 1-year proportional survival (%) | 83 |
| 5-year proportional survival (%)§ | 67 |
Values expressed as median [IQR] or N [%]. †, 6/156 (4%) of patients were categorized as ungradable, and 5/156 (3%) died; ‡, reason of death: massive hemorrhage intra-operative (n=2), multiorgan failure (n=3), unsuccessful reanimation after cardiac arrest (n=3), respiratory failure and sepsis (n=2), fatal brain hemorrhage (n=1), pulmonary necrosis (n=1), pulmonary adenocarcinoma in the donor lung (n=1); §, 32 patients were censored, 5-year survival not yet reached. ECLS, extracorporeal life support; DBD, donation after brain death; DCD, donation after circulatory death; BMI, body mass index; CMV, cytomegalovirus; D+, seropositive donor; R−, seronegative recipient; BSA, body surface area; LTx, lung transplantation; COPD, chronic obstructive pulmonary disease; CLAD-BOS, chronic lung allograft dysfunction-bronchiolitis obliterans syndrome; CLAD-RAS, chronic lung allograft dysfunction-restrictive allograft syndrome; PAP, pulmonary artery pressure; US, ultrasound; RHC, right heart catheterization; ICU, intensive care unit; PGD, primary graft dysfunction; INR, international normalized ratio; IQR, interquartile range.
Post-operatively, PGD-3 within 72 hours occurred in 72/156 (47%) of patients and 35/156 (22%) were graded PGD-3 at 72 hours post-LTx. Ninety-day mortality was 13/156 (8%) including 2 patients who died during surgery. CLAD developed in 37/156 (24%) of patients. One- and 5-year patient survival were 83% and 67%, respectively. Median follow-up post-LTx was 4.5 (IQR, 1.8–8.5) years.
ECLS initiation and duration
LTx procedure and ECLS-related characteristics are summarized in Table 3. Total ischemic time of right and left lung were 431 (IQR, 346–510) and 502 (IQR, 353–601) minutes, respectively. Median anastomosis time was 75 (IQR, 62–88) minutes per lung.
Table 3
| Variables | Bridge to LTx (n=25) | Intra-operative ECLS (n=130) | Post-operative ECLS (n=1) | Total (n=156) |
|---|---|---|---|---|
| LTx procedure | ||||
| LTx type: SL | 0 | 4 [3] | 0 | 4 [3] |
| LTx type: SSL | 24 [96] | 117 [90] | 1 [100] | 142 [91] |
| LTx type: lobar | 1 [4] | 9 [7] | 0 | 10 [6] |
| Thoracotomy | ||||
| Anterior | 21 [84] | 77 [59] | 1 [100] | 99 [63] |
| Clamshell | 4 [16] | 53 [41] | 0 | 57 [37] |
| EVLP use | 0 | 5 [4] | 0 | 5 [3] |
| Total ischemic time RL (min) | 392 [357–518] | 448 [343–509] | 278 | 431 [346–510] |
| Total ischemic time LL (min) | 551 [479–617] | 484 [340–601] | 512 | 502 [353–601] |
| Anastomosis time RL (min) | 86 [74–92] | 74 [62–87] | 74 | 75 [64–88] |
| Anastomosis time LL (min) | 77 [70–94] | 74 [61–85] | 88 | 75 [62–87] |
| Bridge to LTx | ||||
| Awake | 11 [44] | 11 [7] | ||
| Sedated | 14 [56] | 14 [9] | ||
| ECLS planned | 0 | 30 [23] | 0 | 30 [19] |
| ECLS indication | ||||
| Ventilatory or oxygenation problems | 23 [92] | 35 [27] | 1 [100] | 59 [38] |
| Hemodynamic Instability | 2 [8] | 80 [62] | 0 | 82 [53] |
| Surgical complication | 0 | 12 [9] | 0 | 12 [8] |
| Other | 0 | 3 [2] | 0 | 3 [2] |
| ECLS type | ||||
| CPB | 0 | 11 [8] | 0 | 11 [6] |
| VA-ECMO | 1 [4] | 112 [86] | 0 | 113 [72] |
| VV-ECMO | 22 [88] | 6 [5] | 1 [100] | 29 [19] |
| VVA-ECMO | 2 [8] | 1 [1] | 0 | 3 [2] |
| ECLS cannulation | ||||
| Central | 0 | 104 [80] | 0 | 104 [67] |
| Peripheral | 25 [100] | 21 [16] | 1 [100] | 47 [30] |
| Combination | 0 | 1 [1] | 0 | 1 [1] |
| Missing values | 0 | 4 [3] | 0 | 4 [3] |
| Leg cannula | 0 | 4 [3] | 0 | 4 [3] |
Values expressed as median [IQR] or N [%]. ECLS, extracorporeal life support; LTx, lung transplantation; SL, Single lung; SSL, sequential single lung; EVLP, ex-vivo lung perfusion; RL, right lung; LL, left lung; CPB, cardiopulmonary bypass; VA, veno-arterial; VV, veno-venous; VVA, veno-veno-arterial; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range.
ECLS as bridge to transplant
Median duration of ECLS as bridge to LTx (n=25) was 7 (IQR, 5.5–10.2) days. The most common LTx indication in bridged patients was cystic fibrosis in 10/25 (40%), followed by PF 8/25 (32%) and redo-transplant for CLAD 4/25 (16%). All bridged patients were cannulated peripherally and 22/25 (88%) received VV-ECMO. In all cases ECLS was continued intra-operatively, although in 5/25 (20%) the mode of support changed during LTx, with 3/25 (12%) converted from VV- to VA-ECMO, 1/25 (4%) from VV-ECMO to CPB and 1/25 (4%) from VA-ECMO to CPB. Furthermore, in 7/25 (28%) ECLS was prolonged post-operatively.
Intra-operative ECLS
From patients where ECLS was initiated intra-operatively (n=130), 30/130 (23%) were a-priory-planned mostly patients with PAH (13/30; 43%) and PF (9/30; 30%). In 53/130 (41%) clamshell thoracotomy was performed. Most common indication for intra-operative ECLS was hemodynamic instability in 80/130 (62%). VA-ECMO was started in 112/130 (86%), mostly by central cannulation in 104/130 (80%). Analysis of the exact timing of intra-operative ECLS initiation (Table 4) revealed that in 12/130 (9%) cases, ECLS was initiated at a point during surgery after test clamping and pneumonectomy. Notably, in 6/12 (50%) of these cases, ECLS was initiated for surgical complications with bleeding, mostly atrial tear. In the other half of the cases, respiratory or hemodynamic deterioration developed. In 8/12 (67%) cases a conversion from bilateral anterior thoracotomy to clamshell was performed. Median duration of intra-operative ECLS was 302 (IQR, 179–405) minutes.
Table 4
| Timepoint of ECLS initiation | Total (n=130), N [%] |
|---|---|
| Before induction (awake) in OR | 4 [3] |
| After induction or immediately after thoracotomy | 30 [23] |
| Before first pneumonectomy (proof-clamp) | 43 [33] |
| After first pneumonectomy/during first implantation | 6 [5] |
| Before second pneumectomy (proof-clamp) | 39 [30] |
| After second pneumectomy/during second implantation | 6 [5] |
| After second implantation | 2 [2] |
ECLS, extracorporeal life support; OR, operating room.
ECLS post-operative
Post-operative ECLS prolongation was required in 36/130 (28%) cases with a median duration of 2 (IQR, 1–3) days. Only one patient required ECLS post-operatively while transplantation was performed without ECLS. This was due to development of severe PGD.
ECLS-related complications
Annual distribution of different ECLS types and complication rate are illustrated in Figure 3. At least one complication was present in 104/156 (67%) of ECLS cases. The incidence of complications per group and per ECLS mode are plotted in Figure 4A,4B, respectively.


Complications directly related to ECLS occurred in 30/156 (19%) of patients, whereas 87/156 (56%) suffered from indirectly-related complications. In 13/156 (8%) of patients, more than one complication occurred.
Hemothorax requiring surgical revision was the most common complication and occurred mainly in patients with CPB, namely in 5/11 (45%). From the 30 patients requiring continuous renal replacement therapy (CRRT) post-transplant, 6 (20%) suffered from chronic kidney disease prior to LTx and in 13 (43%) ECLS was initiated due to intra-operative hemodynamic instability. Thromboembolism was mostly prevalent in the VV-ECMO group occurring in 6/29 (21%). Vascular complications consisted of air embolus in 4/11 (36%) patients, tear or other mechanical injury in the cannulated vessel in 3/11 (27%), leg compartment syndrome in 2/11 (18%) and leg ischemia in 2/11 (18%). These complications occurred mostly when the arterial circulation was cannulated. From ten patients with cannulation-site related wound complications, 7/10 (70%) developed hematoma requiring surgical intervention and 3/10 (30%) needed a vacuum-assisted therapy. Wound complications occurred mostly in the VV-ECMO group. The most common mechanical complication was presence of clots in the circuit in 4/9 (44%) and cannula thrombus in 3/9 (3%) patients. Neurological complications presented as cerebrovascular bleeding in three patients (CPB or VA-ECMO). On average, patients supported with CPB developed 1.9 complications/case, patients on VV-ECMO 0.76 and VA-ECMO 0.64.
Survival analysis
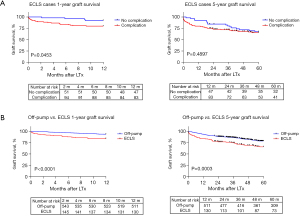
Survival analysis of ECLS patients with vs. without complication (Figure 5A) revealed a significant difference at 1-year (P=0.0453) but comparable 5-year survival (P=0.4897). One- and 5-year patient survival was significantly better in the off-pump compared to ECLS cohort (P<0.0001 and P=0.0003; respectively) (Figure 5B). Median follow-up (off-pump and ECLS cohort) was 5.7 (IQR, 3.1–8.6) years.
Study comparison: patient inclusion criteria and demographic data
Patient inclusion criteria of three recently reported high volume LTx centers (8,14,20) are summarized in Table 5. Whereas two studies also included data from pre-, intra- and post-operative ECLS (14,20), our series is the only describing all modes of extracorporeal support, including CPB. Two centers also included redo-LTx (14,20). Most frequent complications (Table 6) in all studies were revision for hemothorax and acute kidney injury, ranging from 8.8–25% and 8.6-22.8%, respectively. PGD-3 at 72 hours ranged from 1.3–22%.
Table 5
| Study | Years | Number LTx | Number ECLS [%] | Pre-op. ECLS | Intra-op. ECLS | Post-op. ECLS | CPB | VA ECMO | VV ECMO | Central Can. | Peripheral Can. | Redo-LTx | SLTx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orlitová et al. | 2010–2020 | 703 | 156 [22] | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Hoetzenecker et al. (8) | 2010–2016 | 582 | 466 [80] | N/A | √ | √ | N/A | √ | N/A | √ | √ | N/A | N/A |
| Ius et al. (14) | 2010–2019 | 1,161 | 311 [27] | √ | √ | √ | N/A | √ | √ | √ | √ | √ | √ |
| Halpern et al. (20) | 2017–2021 | 237 | 68 [29] | √ | √ | √ | N/A | √ | N/A | √ | N/A | √ | N/A |
√ = included in the study. ECLS, extracorporeal life support; LTx, lung transplantation; Pre-op., pre-operative; Intra-op., intra-operative; Post-op., post-operative; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; VA, veno-arterial; VV, veno-venous; ECMO, extracorporeal membrane oxygenation; Can., cannulation; Redo-LTx, redo-LTx; SLTx, single lung transplantation; N/A, not available/excluded from the study.
Table 6
| Study | Revision hemoth. | AKI | Thromboembolism | Vascular | Wound Can. site | Mechanical | Neurological | PGD-3 0 h |
PGD-3 24 h | PGD-3 48 h | PGD-3 72 h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orlitová et al. | 39/156 [25.0] | 30/156 [19.2] | 22/156 [14.1] | 11/156 [7.1] | 10/156 [6.4] | 9/156 [5.8] | 3/156 [1.9] | 46/156 [29.5] | 40/156 [25.6] | 38/156 [24.4] | 35/156 [22.4] |
| Hoetzenecker et al. (8) | 41/466 [8.8] | 40/466 [8.6] | 13/466 [2.8] | 9/466 [1.9] | N/A | N/A | 2/466 [0.4] | N/A | N/A | N/A | 22/466 [4.7]† |
| Ius et al. (14) | 58/311 [18.6] | 71/311 [22.8] | 2/311 [0.6] | 27/311 [8.7] | 3/311 [1.0] | N/A | 5/311 [1.6] | N/A | 47/311 [15.1] | 48/311 [15.4] | 46/311 [14.8] |
| Halpern et al. (20) | 15/68 [22.1] | 6/68 [8.8] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 28 [16.6]† | |
Values expressed as N [%]. †, calculated/estimated from data available in the manuscript; ‡, PGD-3 at 48 or 72 hours. See Table 1 for definition of complication categories. ECLS, extracorporeal life support; LTx, lung transplantation; PGD-3, primary graft dysfunction grade 3; Revision hemoth., revision for hemothorax; AKI, acute kidney injury; N/A, not available.
When analyzing pooled data of the three other studies (Table 7), our ECLS series includes the largest proportion of pre-operatively intubated patients (14.1% vs. 4.9%, P=0.0001) and the smallest proportion of lobar-LTx (6.4% vs. 13.4%; P=0.0161).
Table 7
| Parameters | Study 1: Orlitová et al. (n=156) | Study 2: Hoetzenecker et al. (n=466) | Study 3: Ius et al. (n=311) |
Study 4: Halpern et al. (n=68) | Pooled data: study 2–4 (n=845) |
P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Study 1 vs. 2 | Study 1 vs. 3 | Study 1 vs. 4 | Study 1 vs. 2–4 | ||||||
| Age (years) | 52 [36–59] | (40.4–45.2)± (15.3–16.2)† |
49 [30–57] | 64 [49–68] | N/A | N/A | N/A | N/A | N/A |
| BMI (kg/m2) | 24.5 [22.8–26.3] | 22±4–9† | 22.1 [18.4–26] | 23.5 [21.1–27.6] | N/A | N/A | N/A | N/A | N/A |
| Female sex | 78 [50.0] | 238 [51.1]† | 169 [54.3] | 30 [44.1] | 437 [51.7] | 0.8534 | 0.3786 | 0.4683 | 0.7276 |
| Pre-intub. | 22 [14.1] | 13 [2.8]† | 28 [9.0] | N/A | 41 [4.9] | <0.0001 | 0.1121 | N/A | 0.0001 |
| Pre-bridge | 25 [16.0] | N/A | 79 [25.4] | 1 [1.5] | 80 [9.5] | N/A | 0.0248 | 0.0011 | 0.0219 |
| LTx indication | |||||||||
| COPD | 29 [18.6] | 127 [27.3] | 14 [4.5] | 12 [17.6] | 153 [18.1] | 0.0329 | <0.0001 | >0.9999 | 0.9101 |
| ILD/PF | 60 [38.5] | 129 [27.7] | 128 [41.2] | 52 [76.5] | 309 [36.6] | 0.0156 | 0.6174 | <0.0001 | 0.0647 |
| CF | 26 [16.7] | 104 [22.3] | 51 [16.4] | 4 [5.9] | 159 [18.8] | 0.1407 | >0.9999 | 0.0326 | 0.5757 |
| PAH | 15 [9.6] | 47 [10.1] | 72 [23.2] | N/A | 119 [14.1] | >0.9999 | 0.0004 | N/A | 0.1587 |
| Redo | 14 [9.0] | N/A | 17 [5.5] | 9 [13.2] | 26 [3.1] | N/A | 0.1693 | 0.3450 | 0.0026 |
| LTx type | |||||||||
| SSLTx | 142 [91.0] | 385 [82.6]‡ | 298 [95.8] | 68 [100] | 751 [88.9] | 0.0102 | 0.0562 | 0.0066 | 0.4846 |
| SLTx | 4 [2.6] | N/A | 13 [4.2] | N/A | 13 [1.5] | N/A | 0.4443 | N/A | 0.3211 |
| Lobar-LTx | 10 [6.4] | 81 [17.4] | 32 [10.3] | N/A | 113 [13.4] | 0.0006 | 0.2293 | N/A | 0.0161 |
Values expressed as median [IQR], mean (± SD) or n [%] according to the design of each study. †, calculated/estimated from data available in the manuscript; ‡, 182 (39%) of lungs underwent size reductio. ECLS, extracorporeal life support; LTx, lung transplantation; BMI, body mass index; Pre-intub., pre-operative intubation; Pre-bridge, pre-operative bridge; COPD, chronic obstructive pulmonary disease or obstructive lung disease; ILD, interstitial lung disease; PF, pulmonary fibrosis; CF, cystic fibrosis; PAH, pulmonary arterial hypertension or other pulmonary vascular disease; Redo, redo LTx; SSLTx, sequential single-lung transplantation; SLTx, single LTx; N/A, data not available; IQR, interquartile range; SD, standard deviation.
Comparing the indications for LTx, it can be noticed from reported series that Hoetzenecker et al. included more chronic obstructive pulmonary disease (COPD) (18.6% vs. 27.3%; P=0.0329) and Halpern et al. significantly more PF patients (38.5% vs. 76.5%; P<0.0001). Ius et al. included proportionally less COPD (18.6% vs. 4.5%; P<0.0001) and more PAH patients (9.6% vs. 23.2%; P=0.0004).
Discussion
This retrospective analysis of 156 ECLS SSLTx patients resulted in an overall ECLS-related complication rate of 67%. Previous studies on ECLS-related complications, reported a lower incidence or described only specific complications. Hoetzenecker et al. reported, in an intra-operative ECMO cohort of 159 patients, on 3.8% of directly ECLS-related complications and 40.4% of other complications (15).
The relatively higher incidence of complications in our series could be related to the detailed description of both directly and indirectly ECLS-related complications as well as the inclusion of CPB. Another important factor is that ECLS in our center is reserved for higher risk patients (e.g., PAH, bridged to LTx, etc.) or patients with intra-operative surgical complications. Interestingly, we observed that patients with ECLS-related complications experienced a decreased 1-year survival when compared to patients not developing any complication. However, this effect disappeared at 5-year follow-up time.
Main complications in our ECLS cohort were revision for hemothorax and acute kidney injury which is in accordance with observations described by Ius et al. (n=311) and Halpern et al. (n=68) (14,20). Furthermore, an association between severe post-operative bleeding in LTx patients and pre- and post-operative ECMO use was previously described by Adelmann et al. (35). In a study focusing on bridged patients only, Kim et al. reported an overall complication incidence of 56% in 100 patients, mostly related to bleeding (36).
Intra-operative use of ECLS during LTx depends on local practices and ranges from 27–100% in different large-volume LTx centers (14-16). Our reported data confirm that a majority (78% in our cohort) of LTx procedures can be performed without ECLS, thereby preventing any potential ECLS-related risks (37). On the other hand, routine ECLS offers intra-operative hemodynamic and respiratory stability with controlled reperfusion of the transplanted lung and decreased right ventricular strain as a counterweight to the risk of ECLS-related complications (38-41). Therefore, comparing LTx outcomes between centers using either of these strategies should be done with caution as recipient demographics and local ECLS practices may substantially differ, as shown in Table 7 (42).
Off-pump LTx strategy requires not only proper hemodynamic and respiratory management with intra-operative re-assessment of ECLS need, but also a meticulous surgical approach (43). As previously reported by our group, it is feasible and safe to use this strategy even in patients undergoing re-transplantation (44). However, it remains essential to identify upfront patients that may strongly benefit from ECLS, such as PAH or patients with severe pulmonary hypertension related to their respiratory condition (9). Our results demonstrate that indeed not all patients have the same baseline need for intra-operative ECLS. In contrast to 94% of PAH patients where ECLS was initiated, it was only required in 8% of COPD patients.
Efforts have been made to predict the need for unplanned intra-operative ECLS use based on recipient-related characteristics (45). In our experience, hemodynamic and respiratory response following test-clamping prior to lung extraction is a reliable assessment tool in predicting ECLS use. Reported data in this study demonstrate that in the majority of ECLS cases, the decision to initiate ECLS was made when clamping was still reversible. This enables ECLS initiation in a non-acute setting and promotes a patient-tailored approach. Only in 12 cases intra-operative ECLS was initiated during lung implantation when reversing of test clamping was not possible anymore. Half of these patients needed ECLS due to surgical complications and the other half due to hemodynamic or respiratory deterioration.
Analysis of complications revealed that more complications occurred when VV-ECMO was used compared to VA-ECMO, which is not in accordance with current literature (28). However, it was also demonstrated that in our cases, peripheral cannulation was predominantly used for VV-ECMO, which might explain the highest chance for developing a cannulation site-related complication. Furthermore, the duration of support might play a role since VV-ECMO was used mainly as a bridge to LTx (median duration of 7 days), whereas VA-ECMO was mainly used for intra-operative support with a median duration of only 302 minutes.
It is well known that ECLS devices are a source of blood trauma and inflammatory activation that compromises the coagulation cascade (46). The complexity of the balance between bleeding and thrombo-embolisation when using ECLS is also reflected in our study. It remains a challenge despite implementing strategies to overcome this, like advances in ECLS technology or close monitoring of coagulation cascade using rotational thrombo-elastometry (47,48). Reported data in this study showed that 19% of ECLS patients required CRRT. Although the cause of kidney failure in these LTx patients is multifactorial, including pre-transplant presence of chronic kidney disease and intra-operative hemodynamic instability, also mechanisms of kidney injury associated with ECLS have been described (33,34).
A major topic of debate remains the association between ECLS and PGD. While Hoetzenecker and colleagues relate a low PGD incidence to standard VA-ECMO use, Loor and colleagues recently published a multicenter international registry showing an association between PGD and VA-ECMO (15,16). Furthermore, a recent single-center prospective observational study showed that levels of post-operative circulating cytokines were significantly higher in ECLS group compared to off-pump group and associated with endothelial cell dysfunction and PGD (49). On the other hand, another recent single-center retrospective study reports on lower leukocyte margination in post-reperfusion biopsies in ECMO group compared to off-pump (50). In our ECLS-series the incidence of PGD-3 within 72 hours was 47% which is higher than the 30.2% previously reported by our center for our overall SSLTx experience (51). This is most probably influenced by our ECLS strategy: either because ECLS could provoke PGD in some cases or because not starting ECLS at the beginning of the procedure might cause fluid accumulation in the transplanted lung resulting in urgent ECLS need during the procedure in some cases. This off-pump strategy might also result in selection of complex cases in the ECLS cohort which is reflected in the finding of the survival analysis between the ECLS and off-pump cohort.
Further prospective and randomized studies are needed to reach more evidence-based consensus on the preferred ECLS strategy for LTx patients. This will also allow to elucidate the role of ECLS in the development of PGD.
Limitations
The main limitation of this study is its retrospective nature that did not allow data collection in a controlled way. Post-operative bleeding leading to hemothorax requiring revision was the most common complication, however, we were unable to retrospectively collect data about the administered blood products as this was not available for the whole study period. Detailed data on renal function were not available. Our study is also limited by the purely descriptive analysis as our ECLS strategy does not allow for comparison or propensity score matching between ECLS and off-pump groups to exclude patient selection bias. The number of patients included in this study is limited by the nature of the off-pump LTx strategy used at our center. Lastly, the generalizability of our results is limited as ECLS practices and demographics of LTx recipients differ substantially among various large volume centers.
Conclusions
Although ECLS remains an essential part of any LTx program, its use is associated with complications that should be considered during the decision process. Larger databases could help to analyze complications and develop better strategies to tailor ECLS to specific patient characteristics and prevent ECLS-related complications in LTx. Further prospective studies are needed to achieve an international consensus on the ECLS use during LTx.
Acknowledgments
The authors would like to thank all members of the Department of Thoracic Surgery and Cardiac Surgery, transplant coordinators, anesthesiologists, intensive care physicians, pulmonologists, cardiologists, and the nursing staff involved in the Leuven Lung Transplant Program, as well as the local network of donor hospitals for their contribution.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ilhan Inci) for the series “Extracorporeal Life Support in Thoracic Surgery” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-443/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-443/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-443/coif). The series “Extracorporeal Life Support in Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. J.D.B. received PhD Fellowship in Fundamental Research from The Research Foundation Flanders (91152820N). L.G. received consulting fees from Biotest and Janssen as well as honoraria for lecture from Janssen, support for attending a meeting from MSD and Biotest and participates on advisory board of Janssen. R.V. received a research grant from Research Foundation Flanders. E.D.T. received predoctoral grant from the University Hospitals Leuven (KOOR-UZ Leuven). D.F.D. received postdoctoral grant from the University Hospitals Leuven (KOOR-UZ Leuven). G.H. received support from Eurosets for attending a meeting. J.W. received Investigator-initiated grant, speakers fee and support for attending a meeting from MSD, Pfizer and Gilead, participates on advisory board of Gilead and received study medication from MSD. A.P.N. received a grant from KU Leuven (C24/18/0730) and support for attending a meeting and speakers fee from Xvivo. L.J.C. is supported by a KU Leuven University Chair funded by Medtronic, a philantropic grant funded by Gunze, a postdoctoral grant from the University Hospitals Leuven (KOOR-UZ Leuven) and a Research foundation Flanders FWO-grant (G090922N). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). There is no experiment in this paper as it is a retrospective study of clinical strategies, therefore informed consent is not required. The study was approved by the local Ethics committee (No. S64384/S51577).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※special series on Extracorporeal Life Support in Thoracic Surgery.
References
- Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021;40:1060-72. [Crossref] [PubMed]
- Ius F, Tudorache I, Warnecke G. Extracorporeal support, during and after lung transplantation: the history of an idea. J Thorac Dis 2018;10:5131-48. [Crossref] [PubMed]
- Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis 2016;8:S353-63. [Crossref] [PubMed]
- Hoetzenecker K, Donahoe L, Yeung JC, et al. Extracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J Thorac Cardiovasc Surg 2018;155:1316-1328.e1. [Crossref] [PubMed]
- Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg 2017;104:412-9. [Crossref] [PubMed]
- Ius F, Natanov R, Salman J, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation may not impact overall mortality risk after transplantation: results from a 7-year single-centre experience. Eur J Cardiothorac Surg 2018;54:334-40. [Crossref] [PubMed]
- Hashimoto K, Hoetzenecker K, Yeung JC, et al. Intraoperative extracorporeal support during lung transplantation in patients bridged with venovenous extracorporeal membrane oxygenation. J Heart Lung Transplant 2018;37:1418-24. [Crossref] [PubMed]
- Hoetzenecker K, Schwarz S, Muckenhuber M, et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg 2018;155:2193-2206.e3. [Crossref] [PubMed]
- Moser B, Jaksch P, Taghavi S, et al. Lung transplantation for idiopathic pulmonary arterial hypertension on intraoperative and postoperatively prolonged extracorporeal membrane oxygenation provides optimally controlled reperfusion and excellent outcome. Eur J Cardiothorac Surg 2018;53:178-85. [Crossref] [PubMed]
- Porteous MK, Ky B, Kirkpatrick JN, et al. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am J Respir Crit Care Med 2016;193:1392-400. [Crossref] [PubMed]
- Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6. [Crossref] [PubMed]
- Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg 2014;98:1936-42; discussion 1942-3. [Crossref] [PubMed]
- Ius F, Aburahma K, Boethig D, et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J Heart Lung Transplant 2020;39:915-25. [Crossref] [PubMed]
- Hoetzenecker K, Benazzo A, Stork T, et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J Thorac Cardiovasc Surg 2020;160:320-327.e1. [Crossref] [PubMed]
- Loor G, Huddleston S, Hartwig M, et al. Effect of mode of intraoperative support on primary graft dysfunction after lung transplant. J Thorac Cardiovasc Surg 2022;164:1351-1361.e4. [Crossref] [PubMed]
- Expert Consensus Panel. The American Association for Thoracic Surgery (AATS) 2022 Expert Consensus Document: The use of mechanical circulatory support in lung transplantation. J Thorac Cardiovasc Surg 2023;165:301-26. [Crossref] [PubMed]
- Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1097-103. [Crossref] [PubMed]
- Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019;38:493-503. [Crossref] [PubMed]
- Halpern SE, Wright MC, Madsen G, et al. Textbook outcome in lung transplantation: Planned venoarterial extracorporeal membrane oxygenation versus off-pump support for patients without pulmonary hypertension. J Heart Lung Transplant 2022;41:1628-37. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Wong JK, Melvin AL, Joshi DJ, et al. Cannulation-Related Complications on Veno-Arterial Extracorporeal Membrane Oxygenation: Prevalence and Effect on Mortality. Artif Organs 2017;41:827-34. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Rupprecht L, Lunz D, Philipp A, et al. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015;7:320-6.
- Glorion M, Mercier O, Mitilian D, et al. Central versus peripheral cannulation of extracorporeal membrane oxygenation support during double lung transplant for pulmonary hypertension. Eur J Cardiothorac Surg 2018;54:341-7. [Crossref] [PubMed]
- Vaquer S, de Haro C, Peruga P, et al. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care 2017;7:51. [Crossref] [PubMed]
- Allou N, Lo Pinto H, Persichini R, et al. Cannula-Related Infection in Patients Supported by Peripheral ECMO: Clinical and Microbiological Characteristics. ASAIO J 2019;65:180-6. [Crossref] [PubMed]
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-76. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-7. [Crossref] [PubMed]
- Nunez JI, Gosling AF, O'Gara B, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med 2022;48:213-24. Erratum in: Intensive Care Med 2022;48:644-5. [Crossref] [PubMed]
- Lorusso R, Barili F, Mauro MD, et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit Care Med 2016;44:e964-72. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, Parise O, et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings From the Extracorporeal Life Support Organization Database. Crit Care Med 2017;45:1389-97. [Crossref] [PubMed]
- Villa G, Katz N, Ronco C. Extracorporeal Membrane Oxygenation and the Kidney. Cardiorenal Med 2015;6:50-60. [Crossref] [PubMed]
- Kielstein JT, Heiden AM, Beutel G, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant 2013;28:86-90. [Crossref] [PubMed]
- Adelmann D, Koch S, Menger J, et al. Risk factors for early bleeding complications after lung transplantation - a retrospective cohort study. Transpl Int 2019;32:1313-21. [Crossref] [PubMed]
- Kim K, Leem AY, Kim SY, et al. Complications related to extracorporeal membrane oxygenation support as a bridge to lung transplantation and their clinical significance. Heart Lung 2022;56:148-53. [Crossref] [PubMed]
- Ius F, Sommer W, Tudorache I, et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J Heart Lung Transplant 2016;35:49-58. [Crossref] [PubMed]
- Bhabra MS, Hopkinson DN, Shaw TE, et al. Controlled Reperfusion Protects Lung Grafts During a Transient Early Increase in Permeability. Ann Thorac Surg 1998;65:187-92. [Crossref] [PubMed]
- Slottosch I, Liakopoulos O, Kuhn E, et al. Controlled lung reperfusion to reduce pulmonary ischaemia/reperfusion injury after cardiopulmonary bypass in a porcine model. Interact Cardiovasc Thorac Surg 2014;19:962-70. [Crossref] [PubMed]
- Andreasson A, Hoetzenecker K. Commentary: Why a routine venoarterial extracorporeal membrane oxygenation support strategy is a good idea in lung transplantation. J Thorac Cardiovasc Surg 2022;164:1363-4. [Crossref] [PubMed]
- Orlitová M, Verbelen T, Frick AE, et al. The hemodynamic interplay between pulmonary ischemia-reperfusion injury and right ventricular function in lung transplantation: a translational porcine model. Am J Physiol Lung Cell Mol Physiol 2023;325:L675-88. [Crossref] [PubMed]
- Chang SH, Henn MC. Commentary: Lung transplant: No support is best. J Thorac Cardiovasc Surg 2022;164:1362-3. [Crossref] [PubMed]
- Perek B, Jemielity M, Tomczyk J, et al. Deep pericardial stitch enables hemodynamically stable exposure of beating heart. Asian Cardiovasc Thorac Ann 2003;11:203-7. [Crossref] [PubMed]
- Jin X, Vanluyten C, Orlitová M, et al. Off-pump lung re-transplantation avoiding clamshell thoracotomy is feasible and safe: a single-center experience. J Thorac Dis 2023;15:5811-22. [Crossref] [PubMed]
- Hinske LC, Hoechter DJ, Schröeer E, et al. Predicting the Necessity for Extracorporeal Circulation During Lung Transplantation: A Feasibility Study. J Cardiothorac Vasc Anesth 2017;31:931-8. [Crossref] [PubMed]
- Doyle AJ, Hunt BJ. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front Med (Lausanne) 2018;5:352. [Crossref] [PubMed]
- Durila M, Vajter J, Garaj M, et al. Rotational thromboelastometry reduces blood loss and blood product usage after lung transplantation. J Heart Lung Transplant 2021;40:631-41. [Crossref] [PubMed]
- Willers A, Arens J, Mariani S, et al. New Trends, Advantages and Disadvantages in Anticoagulation and Coating Methods Used in Extracorporeal Life Support Devices. Membranes (Basel) 2021;11:617. [Crossref] [PubMed]
- Chacon-Alberty L, Ye S, Elsenousi A, et al. Effect of intraoperative support mode on circulating inflammatory biomarkers after lung transplantation surgery. Artif Organs 2023;47:749-60. [Crossref] [PubMed]
- Calabrese F, Pezzuto F, Fortarezza F, et al. Evaluation of Tissue Ischemia/Reperfusion Injury in Lung Recipients Supported by Intraoperative Extracorporeal Membrane Oxygenation: A Single-Center Pilot Study. Cells 2022;11:3681. [Crossref] [PubMed]
- Vandervelde CM, Vos R, Vanluyten C, et al. Impact of anastomosis time during lung transplantation on primary graft dysfunction. Am J Transplant 2022;22:1418-29. [Crossref] [PubMed]


