Prognostic significance of CT-emphysema score in patients with advanced squamous cell lung cancer
Introduction
Chronic obstructive pulmonary disease (COPD) and lung cancer present major medical challenges; COPD is the third leading cause of death and lung cancer is the leading cause of cancer-related death in the United States (1,2). Several studies have established a relationship between COPD and lung cancer. The prevalence of COPD in newly-diagnosed lung cancer patients was estimated at about 50% (3) and COPD is considered an independent risk factor of lung cancer, irrespective of smoking history (4,5).
Emphysema, one of the classic subtypes of COPD, is characterized by abnormal and permanent enlargement of airspaces distal to terminal bronchioles and accompanying destruction of alveolar walls. Computed tomography (CT) is an established method for the detection and quantification of emphysema (6,7), and CT-diagnosed emphysema has also been reported to be associated with an increased risk of lung cancer independently of smoking history and airflow obstruction (8). Furthermore, recent studies have reported that presence of emphysema detected by CT in resected and in all stages of non-small cell lung cancer (NSCLC) patients has an adverse prognostic impact (9,10).
The determination of lung cancer histologic subtype is important for treatment decision making and histologic subtype is considered to have a prognostic role in a metastatic setting (11). Furthermore, the relationship between emphysema and lung cancer appears to depend on histologic subtype. A recent study demonstrated a significantly higher risk of squamous cell carcinoma in the presence of CT detected emphysema than for other histologic subtypes (12). In view of the shared pathogenic mechanisms of emphysema and squamous cell lung cancer (13), we questioned whether the severity of emphysema influences prognosis in patients with advanced stage squamous cell lung cancer. Accordingly, the purpose of our study was to analyze the prognostic value of CT-emphysema score in patients with advanced squamous cell lung cancer that received palliative chemotherapy.
Methods
Study subjects
We retrospectively identified 494 consecutive patients with advanced NSCLC (stages IIIB and IV) that received palliative chemotherapy at Gachon University Gil Medical Center (Incheon, Korea) between January 2010 and December 2014. Of these patients, 96 (19.4%) had histologically proven squamous cell carcinoma. Twelve patients that had undergone pulmonary resection were excluded, and thus, the study cohort consisted of 84 patients. The medical records of all study subjects were reviewed. Patient information included age, sex, performance status (PS), smoking habit, stage, spirometric variables, chemotherapy regimen, tumor response, and survival. Spirometry was performed with a bronchodilator as recommended by American Thoracic Society at time of lung cancer diagnosis. COPD was diagnosed if the ratio of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FEV1/FVC) was lower than 70%. The Global initiative for Chronic Obstructive Lung Disease (GOLD) system was used to classify subjects into mild (GOLD I), moderate (GOLD II) and severe (GOLD III–IV) airflow limitation categories (14). Tumor responses were classified using Response Evaluation Criteria in Solid Tumors (RECIST 1.1). This retrospective study was approved by the Institutional Review Board of our institution and patient confidentiality was maintained throughout the study.
CT-emphysema score
Chest CT scans were obtained using Siemens multi-detector helical CT scanners (SOMATOM Definition or SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany). With a patient in the supine position, scans were performed during full inspiration using the following acquisition parameters: 1 mm collimation, 120–140 kV, 75–350 mA, 0.75–1 s scan time, and 1–2 mm slice thickness. Emphysema severity was determined using CT images obtained at time of diagnosis by a subspecialty-trained chest radiologist using the Goddard scoring system (15). The reader was instructed to divide lungs into six regions: upper, middle, and lower lung fields (upper lung; 1 cm above the superior margin of the aortic arch, middle lung; 1 cm below the carina, lower lung; 3 cm above the top of the diaphragm) on right and left sides. Emphysema is characterized by CT as areas of low attenuation that contrast with the normal attenuation of surrounding lung parenchyma. Lung regions were graded as follows; no emphysema (score 0), ≤25% emphysema (score 1), ≤50% emphysema (score 2), ≤75% emphysema (score 3) and >75% emphysema (score 4). Scores of the six regions were summed to obtain total scores, giving a minimum total score of 0 and a maximum of 24. Total CT-emphysema scores of 0 or 1 were defined as no emphysema, since 95% of nonsmokers in previous series had lungs with <5% emphysematous involvement (10).
Statistical analysis
Pearson’s chi-squared or Fisher’s exact tests were used to compare categorical variables, and continuous variables were compared using the Student’s t-test or Mann-Whitney U test. Relationships between pairs of continuous variables were examined using Pearson’s correlation analysis. Overall survival (OS) was defined as time between treatment commencement and date of death or last follow-up. OSs were estimated using the Kaplan-Meier method and compared using the log-rank test. Maxstat, a maximal chi-square method in open source statistical software R (R Development Core Team, Vienna, Austria, http://www.R-project.org), was used to determine the optimal cutoff point for CT-emphysema score. Multivariable Cox regression analysis (enter method) was performed to identify significant prognostic factors of survival. Variables with P values of <0.1 by univariable analysis were included in the multivariable model, which was adjusted for known prognostic factors (age, stage, and PS). Statistical significance was accepted for P values of <0.05, and the statistical analysis was performed using SPSS for Windows ver. 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics and CT-emphysema scores
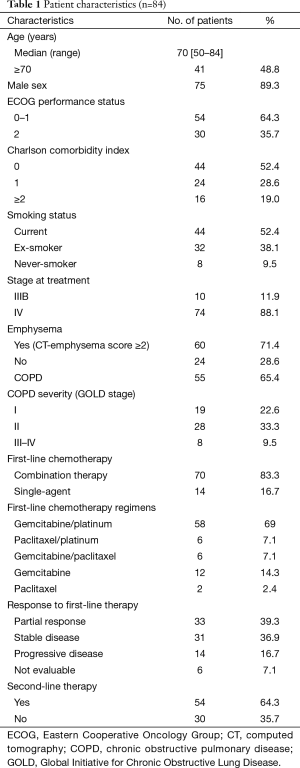
Baseline characteristics for the 84 study subjects are summarized in Table 1. Median patient age was 70 years (range, 50–84 years) and 75 (89.3%) were male. Seventy-six patients (90.5%) had a smoking history and 55 patients (65.4%) had COPD. Combination chemotherapy was administered to 70 patients (83.3%), and 33 patients (39.3%) responded to first-line chemotherapy.
Full table
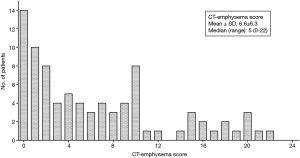
Emphysema, defined as a CT-emphysema score ≥2, was present in 60 patients (71.4%), and the mean CT-emphysema score was 6.6±6.3 (median, 5; range, 0–22; interquartile range, 9) (Figure 1). All of them had diffuse emphysema across multiple regions, and there was no patient with localized emphysema (i.e., one area with >75% emphysema and no emphysema in other areas). Seven patients (8.3%) had combined pulmonary fibrosis and emphysema (CPFE).
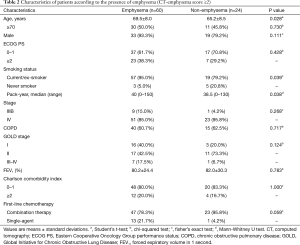
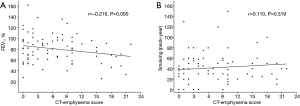
The presence of emphysema (CT-emphysema score ≥2) was found to be significantly associated with smoking history (P=0.039) and higher smoking pack-years (P=0.038). However, no significant differences was observed between patients with or without emphysema in terms of sex, age, PS, stage, COPD, and FEV1 (%) (Table 2). CT-emphysema score was not found to be significantly related to FEV1 (r=−0.215; P=0.050) or smoking pack years (r=0.110; P=0.319) (Figure 2).
Full table
Survival analysis
Over a median follow-up of 7.8 months [95% confidence interval (CI), 4.2–11.3 months], 76 patients (90.5%) died, and median OS was 9.1 months (95% CI, 5.7–12.4 months).
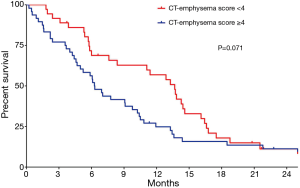
Using the maximal chi-square method to determine an optimal cutoff for CT-emphysema score, patients with a score of ≥4 showed a tendency toward poorer OS than patients with a score of <4 (6.3 vs. 13.7 months; P=0.071 by the log-rank test; Figure 3).
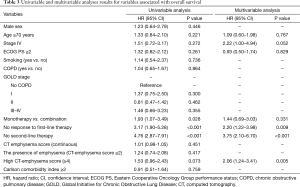
Univariable and multivariable analyses results for prognostic factors of OS are summarized in Table 3. Univariable analysis showed that single-agent chemotherapy (P=0.028), no response to first-line therapy (P<0.001), and no second-line treatment (P<0.001) were significantly associated with poor OS, and that the presence of COPD and COPD severity were not significant prognostic factors. However, multivariable analysis showed that higher CT-emphysema score (≥4) was an independent prognostic factor for shorter OS [hazard ratio (HR) =2.06; 95% CI, 1.24–3.41; P=0.005], along with no response to first-line therapy (HR =2.20; 95% CI, 1.22–3.98; P=0.009) and no second-line therapy (HR =3.75; 95% CI, 2.10–6.70; P<0.001).
Full table
Discussion
The majority of NSCLC patients is initially diagnosed with advanced stage and generally requires systemic therapy (16). Squamous cell carcinoma is the second most common histology in NSCLC, and accounts for approximately 30% of all cases (17). The sub-classification of NSCLC as squamous or non-squamous is important for treatment decision making in the setting of advanced disease, because underlying driving genetic abnormalities, such as, epidermal growth factor receptor mutations and anaplastic lymphoma kinase gene rearrangements, have been associated with a non-squamous rather than a squamous histology (18). Furthermore, squamous cell lung cancer is highly prevalent in the smoking population, and smoking is the common causative factor of emphysema. Therefore, in the present study, we investigated the prognostic significance of CT-determined emphysema severity in patients with advanced squamous cell lung cancer. It was found a higher CT-emphysema score was significantly associated with shorter OS after adjusting for other prognostic factors, including age, stage, PS, chemotherapy (single agent vs. doublet), response to first-line therapy, and second-line treatment (yes vs. no).
We observed that advanced squamous cell lung cancer is frequently accompanied by emphysema (CT-emphysema score ≥2) (71.4%). As compared with previous studies, which reported a prevalence of emphysema of 58% in clinical stage I NSCLC and of 31% in all stages of NSCLC patients (9,10), the higher percentage observed in the present study was probably caused by the inclusion of a greater proportion of patients with an advanced stage or a squamous cell histology.
In the present study, COPD (as defined by airflow limitation) was not found to be associated with poor prognosis in advanced squamous cell lung cancer. This finding is consistent with previous studies, which also found COPD had no prognostic impact in patients with early-stage NSCLC or in patients with advanced NSCLC that received chemotherapy (10,19). In addition, the severity of emphysema in the present study was not well correlated with FEV1 (%), which was also reported by Ueda et al. (10). COPD is a heterogeneous syndrome with variable contributions from emphysema, chronic bronchitis, and small airway disease, and is traditionally categorized using spirometric measures, such as, FEV1 (14). For this reason, COPD severity fails to reflect disease heterogeneity, because spirometry results reflect airflow obstruction caused by combinations of these disease processes.
One plausible mechanism for the poor prognosis associated with emphysema in squamous lung cancer concerns the relationship between the tumor microenvironment and the clinicopathologic aggressiveness of the cancer (20). Matrix metalloproteinase (MMP)-2, which is up-regulated in emphysematous lungs (21), has been shown to play important roles in tumor progression (22). In a previous study, it was reported that strong stromal MMP-2 expression was a significant poor prognostic factor in squamous cell lung cancer, but not in lung adenocarcinoma (23). Another explanation is that emphysema may confer a risk for chemotherapy-related severe adverse events, for example, emphysema by chest CT has been identified to be a risk factor of severe pneumonia (24). Similarly, in the present study, treatment-associated mortality resulting from pneumonia was more frequent in patients with a higher CT-emphysema score (16.7% vs. 8.3%).
The Goddard semi-quantitative scoring system was used in the present study to evaluate the severity of emphysema in baseline CT scans. This visual scoring method is straightforward and can be performed rapidly because it does not require post-processing techniques, such as, lung segmentation, thresholding, or manual extraction for quantitative CT assessment (25). Furthermore, previous studies have reported good agreement of semi-quantitative scores between expert readers for the assessment of presence and extent of emphysema, and subjective visual assessments were found to be well correlated with objective lung attenuation measurements obtained by two- or three-dimensional CT densitometry (25-27). Since all lung cancer patients undergo an initial chest CT scan for diagnosis and staging, this method might allow the routine quantitative assessment of emphysema in lung cancer patients without additional cost or radiation exposure.
The present study has several limitations that require consideration. First, the number of patients included was small, although the study populations are homogeneous in terms of histology, stage, and treatment. Second, we did not evaluate the prognostic significance of CPFE due to small number of patients (n=7), although a previous report showed patients with CPFE showed poor prognosis than those with emphysema only (28). Furthermore, we cannot analyze the value of the emphysema distribution (i.e., one area with >75% emphysema vs. diffuse emphysema across multiple regions) because no patient had localized emphysema. Finally, detailed information regarding the smoking status after cancer diagnosis cannot be obtained since this study was conducted in retrospective manner. It has been reported that smoking status after cancer diagnosis affects prognostic outcomes (29,30).
In conclusion, a higher CT emphysema score, as determined using baseline CT scans, was found to be associated with poor prognosis in patients with advanced squamous cell lung cancer. Furthermore, our findings suggest CT-emphysema score could be used to better predict prognosis in advanced squamous cell lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of our institution.
References
- Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep 2012;61:1-51. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 34:380-6. [Crossref] [PubMed]
- Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One 2011;6:e17479. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738-44. [Crossref] [PubMed]
- Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT—comparison with macroscopic and microscopic morphometry. Radiology 2006;238:1036-43. [Crossref] [PubMed]
- Shaker SB, Dirksen A, Bach KS, et al. Imaging in chronic obstructive pulmonary disease. COPD 2007;4:143-61. [Crossref] [PubMed]
- Smith BM, Pinto L, Ezer N, et al. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer 2012;77:58-63. [Crossref] [PubMed]
- Gullón JA, Suárez I, Medina A, et al. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer 2011;71:182-5. [Crossref] [PubMed]
- Ueda K, Jinbo M, Li TS, et al. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res 2006;12:6730-6. [Crossref] [PubMed]
- Cetin K, Ettinger DS, Hei YJ, et al. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol 2011;3:139-48. [Crossref] [PubMed]
- Smith BM, Schwartzman K, Kovacina B, et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer 2012;76:61-6. [Crossref] [PubMed]
- Yang P, Sun Z, Krowka MJ, et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med 2008;168:1097-103. [Crossref] [PubMed]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. [Crossref] [PubMed]
- Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33:379-87. [Crossref] [PubMed]
- Chansky K, Sculier JP, Crowley JJ, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009;4:792-801. [Crossref] [PubMed]
- Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669-92. [Crossref] [PubMed]
- Cheng H, Shcherba M, Kandavelou K, et al. Emerging drugs for squamous cell lung cancer. Expert Opin Emerg Drugs 2015;20:149-60. [Crossref] [PubMed]
- Izquierdo JL, Resano P, El Hachem A, et al. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int J Chron Obstruct Pulmon Dis 2014;9:1053-8. [Crossref] [PubMed]
- Murakami J, Ueda K, Sano F, et al. Pulmonary emphysema and tumor microenvironment in primary lung cancer. J Surg Res 2016;200:690-7. [Crossref] [PubMed]
- Ohnishi K, Takagi M, Kurokawa Y, et al. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 1998;78:1077-87. [PubMed]
- Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011;278:16-27. [Crossref] [PubMed]
- Ishikawa S, Takenaka K, Yanagihara K, et al. Matrix metalloproteinase-2 status in stromal fibroblasts, not in tumor cells, is a significant prognostic factor in non-small-cell lung cancer. Clin Cancer Res 2004;10:6579-85. [Crossref] [PubMed]
- Eom JS, Song WJ, Yoo H, et al. Chronic obstructive pulmonary disease severity is associated with severe pneumonia. Ann Thorac Med 2015;10:105-11. [Crossref] [PubMed]
- Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology 1999;211:541-7. [Crossref] [PubMed]
- Muller NL, Staples CA, Miller RR, et al. "Density mask". An objective method to quantitate emphysema using computed tomography. Chest 1988;94:782-7. [PubMed]
- Litmanovich D, Boiselle PM, Bankier AA. CT of pulmonary emphysema--current status, challenges, and future directions. Eur Radiol 2009;19:537-51. [Crossref] [PubMed]
- Usui K, Tanai C, Tanaka Y, et al. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology 2011;16:326-31. [Crossref] [PubMed]
- Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [Crossref] [PubMed]
- Sitas F, Weber MF, Egger S, et al. Smoking cessation after cancer. J Clin Oncol 2014;32:3593-5. [Crossref] [PubMed]