
Prognostic factors in lung transplantation after extracorporeal membrane oxygenation bridging therapy: a systematic review and meta-analysis
Highlight box
Key findings
• Prolonged extracorporeal membrane oxygenation (ECMO) support, declining liver and kidney functions, and complications during ECMO are vital prognostic factors in lung transplantation following ECMO bridging therapy.
What is known and what is new?
• Prior cohort and case-control studies indicate significant improvements in the prognosis of lung transplant patients undergoing ECMO bridging therapy. Clinical data on the prognostic factors of ECMO as a bridging therapy for lung transplantation have shown inconsistency.
• This manuscript contributes a systematic review of prognostic factors in lung transplant recipients following ECMO bridging therapy.
What is the implication, and what should change now?
• This systematic review and meta-analysis can inform the establishment of standardized nursing protocols and the development of an evaluation index system for ECMO patient care.
Introduction
In recent decades, lung transplantation has emerged as the definitive treatment for end-stage lung disease. However, a critical limitation to its rapid advancement is the scarcity of donors (1,2). Currently, there is a notable disparity between the limited availability of donors and the excess of patients awaiting transplantation, leading to prolonged waiting times and a consequent high mortality rate during this period (3-5). A 2023 analysis from the United Network for Organ Sharing database reveals that, although the case fatality rate during the waiting period at lung transplant centers in the United States has declined compared to previous years, it remains alarmingly high at 23% (6).
Confronted with this challenge, medical professionals worldwide are focused on enhancing the survival rates of patients with end-stage lung disease during the transplant waiting period. In the past several decades, extracorporeal membrane oxygenation (ECMO) systems have experienced significant advancements (7,8). The primary goal of employing ECMO as a bridging therapy before lung transplantation is to prevent the deterioration of the patients’ physical condition and to improve their overall health and resilience, thereby creating optimal conditions for successful post-transplant outcomes (9). These improvements have led to the growing use of ECMO as a preferred bridging therapy over tracheal intubation for patients with cardiopulmonary failure, primarily to avoid hospital-acquired pneumonia, ventilator-refractory hypoxia and to maintain walking ability and participation in preoperative rehabilitation (10-12). However, despite these advancements, ECMO usage carries inherent risks, with the duration of use correlating to an increased likelihood of complications (13,14).
Despite existing research exploring the prognostic factors associated with ECMO as a bridging therapy before lung transplantation, these studies often lack consistency, and their findings vary (15-17). In response to this gap, our research reviews prognostic factors related to lung transplantation following ECMO bridging therapy. This analysis aims to provide robust evidence supporting the efficacy of ECMO bridging in enhancing the prognosis of lung transplant patients. We present this article in accordance with the MOOSE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1709/rc) (18).
Methods
Protocol and guidelines
The protocol for this review is registered with PROSPERO (CRD42023453709).
Inclusion criteria
Eligible trials include those that enroll adult patients (age ≥18 years) undergoing lung transplantation bridging ECMO, focus on risk factors affecting prognosis, provide information on all-cause (non-accidental) mortality, or report specific causes separately, and are case-control or cohort studies.
Exclusion criteria
This study excludes case reports or case series, studies where only abstracts are available or the full text is inaccessible, and studies with data that cannot be converted or applied.
Outcomes
The primary outcome is the mortality rate, with the secondary outcome being risk factors.
Search strategy
One author (Y.Z.) searched databases such as PubMed, Cochrane Library, Embase, CINAHL, Web of Science, Scopus, and ProQuest from their inception until August 11, 2023 (Tables S1-S6). Searches were also performed on ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform to identify ongoing and unpublished trials meeting our inclusion criteria. We also reviewed the reference lists of identified trials and systematic reviews to ensure thoroughness. There were no language restrictions imposed.
Study selection
Duplicate records were removed, after which two independent researchers (Y.Z. and J.S.Y.L.) screened all titles and abstracts. Studies meeting the initial eligibility criteria had their full texts obtained for further scrutiny. Discrepancies between researchers were resolved by consensus.
Data collection process
Two independent researchers (Y.Z. and J.S.Y.L.) employed a standardized data extraction form to gather data from the included trials. Extracted details encompassed author, publication year, study location, study design, sample size, and risk factors. A third researcher settled any disagreements.
Assessment of risk of bias and quality of evidence
The quality of the included trials was independently evaluated by two researchers (Y.Z. and J.S.Y.L.) using the Newcastle-Ottawa Scale (19). This scale assesses study population selection, group comparability, and exposure factors. A total score of six or more was deemed indicative of high-quality literature.
Statistical analysis
Statistical analyses were conducted using RevMan (version 5.4) and STATA (version 17.0). Odds ratio and their 95% confidence intervals (CIs) were used to assess outcomes, considering a P value of less than 0.05 as statistically significant. Heterogeneity was evaluated using the I2 test (I2<50%), fixed (20). Fixed effects models were used to combine the outcomes. However, random effects models were utilized if significant heterogeneity was present (I2≥50%). The presence of small study effects was assessed both qualitatively, through funnel plot inspection, and quantitatively, using Egger’s test (21).
Sensitivity analyses
Sensitivity analyses were executed using the following methodology: each paper was individually removed from the dataset, and the resultant impact on the combined effect size (ES) was assessed to determine the influence of each study on the overall meta-analysis.
Results
Eligible studies and study characteristics
The initial search yielded 12,886 records, culminating in the inclusion of eight eligible trials (22-29) in the final meta-analysis (Figure 1). The methodological quality evaluation scores of these eight studies were six points or higher, indicating high quality. The characteristics of the included trials are summarized in Table 1.
Table 1
| Studies | Country | Study design | Year | Sample size | Mortality rate (%) | Risk factors |
|---|---|---|---|---|---|---|
| Neumann (22) | Switzerland | Case-control | 2023 | 221 | 29.40 | Age, newly detected liver failure, red blood cell transfusion, platelet concentrate transfusion |
| Kim (23) | South Korea | Case-control | 2022 | 100 | 27.50 | Intracranial hemorrhages, RRT use, bloodstream infection occurrence |
| Minqiang (24) | China | Case-control | 2021 | 267 | NR | Delayed withdrawal ECMO |
| Kim (25) | South Korea | Cohort | 2021 | 64 | 53.80 | Sedated BTT ECMO |
| Weig (26) | German | Case-control | 2013 | 26 | 46.20 | SOFA score lower, maximal bilirubin, bilirubin prior to transplantation |
| Crotti (27) | Italian | Case-control | 2013 | 25 | 24 | Waiting time on ECMO (up to 14 days or longer), invasive mechanical ventilation |
| Oh (28) | Korean | Case-control | 2021 | 41 | 55 | Long-term ECMO support (14 days) |
| Hayanga (29) | American | Case-control | 2016 | 342 | 26.90 | Low-volume centers |
RRT, renal replacement therapy; NR, not reported; ECMO, extracorporeal membrane oxygenation; BTT, bridge to transplant; SOFA, sequential organ failure assessment.
First outcome: mortality rate
Seven of the studies reported the mortality rate associated with ECMO support as a bridge to lung transplantation. Due to heterogeneity among these studies, a random effect model was employed for analysis. The pooled was 37% (ES 0.37, 95% CI: 0.28–0.46, I2=83.57%, Figure 2). The mortality rate for ECMO support as a bridge to lung transplantation was found. This high rate underscores the need for further investigation into prognostic factors. Sensitivity analysis indicated minimal impact on the combined ES when any single study was excluded. The funnel plot analysis suggested symmetry, and Egger’s tests revealed no significant minor study effects (Figure S1).

Secondary outcome: risk factors
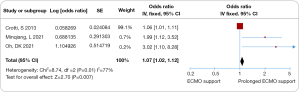
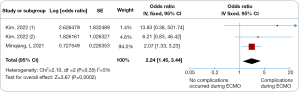
All eight trials investigated various risk factors. The prognosis for patients undergoing lung transplantation with ECMO bridging was found to be significantly influenced by several key factors. Prolonged ECMO support in lung transplantation bridging was associated with increased mortality (odds ratio 1.07, 95% CI: 1.02–1.12, I2=77%, Figure 3). Additionally, deterioration in liver and kidney function was linked to a higher risk of mortality (odds ratio 3.62, 95% CI: 2.37–5.54, I2=0%, Figure 4), as were complications occurring during ECMO (odds ratio 2.24, 95% CI: 1.45–3.44, I2=5%, Figure 5). Notable heterogeneity was observed among the studies. Sensitivity analysis indicated that excluding any individual study had minimal impact on the overall ES. The funnel plot analysis demonstrated symmetry, and Egger’s tests found no significant minor study effects (Figures S2-S4).

Discussion
In this meta-analysis involving 1,086 participants across eight trials, the mortality rate for ECMO support as a bridge to lung transplantation was 37% (ES 0.37, 95% CI: 0.28–0.46). The prognosis for patients undergoing lung transplantation with ECMO bridging was notably influenced by factors like extended ECMO support duration (odds ratio 1.07, 95% CI: 1.02–1.12, I2=77%), worsening of liver and kidney functions (odds ratio 3.62, 95% CI: 2.37–5.54, I2=0%), and complications during ECMO (odds ratio 2.24, 95% CI: 1.45–3.44, I2=5%). The findings suggest that longer ECMO support, deteriorating liver and kidney function, and complications during ECMO in lung transplant patients are indicative of a poorer prognosis.
Principal findings and their comparison with other studies
The observed mortality rate in this study aligns with findings from two prior systematic reviews (30,31). An earlier meta-analysis revealed that patients receiving long-term ECMO-supported lung transplantation as bridging therapy had a worse prognosis compared to those not receiving ECMO. However, this gap has narrowed significantly between 2009 and 2011 (31). Over the last decade, there have been remarkable improvements in the prognosis of patients undergoing ECMO bridging therapy. Hayanga et al. (32) noted that the 1-year survival rate for patients who underwent lung transplantation after ECMO bridging between 2000 and 2002 was 25%, increasing to as high as 74% between 2009 and 2011. This significant enhancement is attributed to advanced ECMO system design, improved patient care during bridging, and more effective patient selection (5,33,34).
The mortality rate was significantly higher in trials with prolonged ECMO support. With advancements in extracorporeal life support, ECMO as a bridge for lung transplantation has become crucial for intraoperative and postoperative circulatory support, sustaining recipients during the lung transplantation window period (35). Patients requiring extended ECMO support exhibited a 1.07-fold increase in post-transplant mortality. Such patients are typically critically ill, often requiring combined mechanical ventilation, which may exacerbate organ damage during acute recovery (35,36). Recipients needing longer ECMO support tend to have more extended hospital stays and poorer physical conditions, suggesting that prolonged ECMO may be a key predictor of outcomes in these patients.
A key observation from our analysis is the significantly higher mortality in trials involving patients with deteriorated liver and kidney function. Complications such as acute kidney injury and new liver damage frequently occur during ECMO treatment (37). Fluid overload, a common issue in ECMO patients, often necessitates renal replacement therapy (RRT) to maintain fluid balance and metabolic control, a practice widely implemented across different centers (38). The association of liver and kidney function deterioration with increased mortality (odds ratio 3.62, 95% CI: 2.37–5.54) underscores the importance of comprehensive care. Management of patients undergoing ECMO for lung transplantation should involve a multidisciplinary team, including the establishment of an anticoagulation nursing team and integrated medical care for dynamic management. Such a collaborative approach allows for timely adjustments in diagnosis and treatment, ensuring patient safety.
ECMO’s broad application brings with it a range of complications during patient care, including infections, bleeding, thrombosis, hemolysis, renal injury, and hepatic impairment. The balance between the risks and benefits of ECMO should be carefully considered in clinical practice. Our review found a significant increase in mortality in trials involving patients who experienced complications during ECMO (odds ratio 2.24, 95% CI: 1.45–3.44). Notably, complications such as intracranial hemorrhages and bloodstream infections were strongly linked to mortality. ECMO assistance elevates the risk of bleeding, thrombosis, and infections, all of which can adversely affect patient prognosis (13,23). Patients may experience blood pressure fluctuations and excessive bleeding during surgery. Post-transplantation, pulmonary artery pressure often remains elevated compared to normal levels (39,40). Inadequate removal of ECMO support can lead to hemodynamic instability (41). Therefore, this study emphasizes the criticality of patient selection, particularly in evaluating perioperative tolerance to ECMO and its associated complications. The optimal utilization of ECMO, coupled with continuous assessment of patients during bridge therapy, is vital to determining their lung transplantation eligibility.
Strengths and limitations
This systematic review and meta-analysis boasts several methodological strengths. We strictly adhered to the Cochrane Collaboration guidelines and the MOOSE statement, including the implementation of a pre-defined protocol. Additionally, the Newcastle-Ottawa Scale was employed to rigorously assess the quality of evidence, which was found to be high for the primary outcome. Our study provides a comprehensive review of the current data on this subject. Nonetheless, our study is not without limitations. One significant issue is the variability in the definitions of certain risk factors. Different interpretations of specific risks and/or the duration of ECMO support could lead to heterogeneity in risk estimates. Consequently, our analysis could not fully explore the interplay between various risks.
At present, there are a limited number of studies focusing on the risk factors affecting the prognosis of patients undergoing lung transplantation with ECMO bridging. The relatively small number of studies in this analysis restricts our ability to evaluate related risk factors comprehensively. Therefore, future research should involve large-scale, high-quality prospective studies to better understand and identify these risk factors.
Implications
The current lack of a standardized approach for the appropriate withdrawal of ECMO represents a significant gap in clinical practice. Additionally, there is a scarcity of studies exploring patient prognosis post-successful ECMO withdrawal, as well as a deficiency in long-term clinical follow-up data. Enhancing the withdrawal process and developing a predictive scoring system for successful withdrawal outcomes are crucial for maximizing the potential of ECMO technology in future clinical applications.The management of patients receiving both ECMO and RRT is notably complex and demands highly skilled nursing staff. Standardization in nursing practices for this combined therapy is lacking, as are unified nursing evaluation criteria. The clinical application of ECMO and RRT combined is still exploratory, with most existing studies deriving from empirical observations. Future research should evaluate various nursing interventions during ECMO via employing diverse nursing models and multidisciplinary approaches to generate substantial-high-quality data.
Advancing ECMO nursing scientifically necessitates the establishment of standardized nursing protocols and the development of an evaluation index system specific to ECMO nursing. Such initiatives will play a pivotal role in minimizing ECMO-related complications, enhancing the quality of nursing care, and improving the survival rates of critically ill patients. Consequently, this will lead to improved prognoses and outcomes for these patients.
Conclusions
Overall, this meta-analysis found the mortality rate associated with ECMO bridging in lung transplantation to be 37%, influenced by factors such as prolonged ECMO support, declining liver and kidney function, and complications during ECMO. The prognosis for lung transplant recipients is adversely impacted by longer ECMO support durations, deteriorating liver and kidney functions, and ECMO-related complications. Nevertheless, there is a significant opportunity for caregivers to improve outcomes. By proactively identifying and managing these risk factors through early intervention, the mortality rate among lung transplant recipients can potentially be reduced. This approach emphasizes the importance of vigilant monitoring and timely response to changes in patient conditions during the ECMO bridging process.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1709/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1709/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1709/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sabashnikov A, Patil NP, Popov AF, et al. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysis†. Eur J Cardiothorac Surg 2016;49:46-53. [Crossref] [PubMed]
- Hayes D Jr, Tobias JD, Tumin D. Center Volume and Extracorporeal Membrane Oxygenation Support at Lung Transplantation in the Lung Allocation Score Era. Am J Respir Crit Care Med 2016;194:317-26. [Crossref] [PubMed]
- Campo-Canaveral De La Cruz JL, Dunne B, Lemaitre P, et al. Deceased-donor lobar lung transplant: A successful strategy for small-sized recipients. J Thorac Cardiovasc Surg 2021;161:1674-85. [Crossref] [PubMed]
- Barbero C, Messer S, Ali A, et al. Lung donation after circulatory determined death: a single-centre experience. Eur J Cardiothorac Surg 2019;55:309-15. [Crossref] [PubMed]
- Furfaro D, Aversa M, Shah L, et al. Low Lung Allocation Score Predicts Poor Waitlist Outcomes for Patients on Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation. J Heart Lung Transplant 2020;39:S217-8.
- Deitz RL, Emerel L, Chan EG, et al. Waitlist Mortality and Extracorporeal Membrane Oxygenation Bridge to Lung Transplant. Ann Thorac Surg 2023;116:156-62. [Crossref] [PubMed]
- Inci I, Ehrsam JP, Van Raemdonck D, et al. Extracorporeal life support as a bridge to pulmonary retransplantation: prognostic factors for survival in a multicentre cohort analysis. Eur J Cardiothorac Surg 2022;61:405-12. [Crossref] [PubMed]
- Chinese Society of Cardiothoracic and Vascular Anesthesiology, Chinese Society of Anesthesiology, Chinese Association of Anesthesiologists, Chinese Thoracic Society, Chinese Society of Critical Care Medicine. Expert Consensus on the Clinical Application of Adult Extracorporeal Membrane Oxygenation under Different Conditions (2020 Edition). Chinese Circulation Journal 2020;35:1052-63.
- Keshavamurthy S, Bazan V, Tribble TA, et al. Ambulatory extracorporeal membrane oxygenation (ECMO) as a bridge to lung transplantation. Indian J Thorac Cardiovasc Surg 2021;37:366-79. [Crossref] [PubMed]
- Wang D, Li X, Xuan C, et al. Analysis of Risk Factors of Prolonged Mechanical Ventilation after Lung Transplantation. Organ Transplantation 2022;13:797-802.
- Hayanga JWA, Hayanga HK, Holmes SD, et al. Mechanical ventilation and extracorporeal membrane oxygenation as a bridge to lung transplantation: Closing the gap. J Heart Lung Transplant 2019;38:1104-11. [Crossref] [PubMed]
- Vela MM, Saez DG, Mohite P, et al. Mid-Term Results of Lung Transplantation (Ltx) after Bridging with Extracorporeal Membrane Oxygenation - Influence of Concomitant Invasive Mechanical Ventilation (Imv). J Heart Lung Transplant 2019;38:S167-8.
- Adelmann D, Koch S, Menger J, et al. Risk factors for early bleeding complications after lung transplantation - a retrospective cohort study. Transpl Int 2019;32:1313-21. [Crossref] [PubMed]
- Cho S, Lee JE, Choi BJ, et al. Risk factors for neuromuscular complications in lower limbs after lung transplantation. Front Neurol 2022;13:1066104. [Crossref] [PubMed]
- Nasir BS, Klapper J, Hartwig M. Lung Transplant from ECMO: Current Results and Predictors of Post-transplant Mortality. Curr Transplant Rep 2021;8:140-50. [Crossref] [PubMed]
- Mulvihill MS, Yerokun BA, Davis RP, et al. Extracorporeal membrane oxygenation following lung transplantation: indications and survival. J Heart Lung Transplant 2017;37:259-67. [Crossref] [PubMed]
- Li LJ, Xu HY, Wang XW, et al. Impact of delayed veno-venous extracorporeal membrane oxygenation weaning on postoperative rehabilitation of lung transplantation: a single-center comparative study. J Artif Organs 2023;26:303-8. [Crossref] [PubMed]
- Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg 2021;156:787-8. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Irwig L, Macaskill P, Berry G, et al. Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ 1998;316:470-author reply 470-1.
- Neumann E, Sahli SD, Kaserer A, et al. Predictors associated with mortality of veno-venous extracorporeal membrane oxygenation therapy. J Thorac Dis 2023;15:2389-401. [Crossref] [PubMed]
- Kim K, Leem AY, Kim SY, et al. Complications related to extracorporeal membrane oxygenation support as a bridge to lung transplantation and their clinical significance. Heart Lung 2022;56:148-53. [Crossref] [PubMed]
- Minqiang L, Xiaoshan L, Bo X, et al. A Retrospective Analysis for Risk Factors and Early Prognosis of Delayed Withdrawal Extracorporeal Membrane Oxygenation After Lung Transplantation. Transplantation 2021;105:867-75. [Crossref] [PubMed]
- Kim NE, Woo A, Kim SY, et al. Long- and short-term clinical impact of awake extracorporeal membrane oxygenation as bridging therapy for lung transplantation. Respir Res 2021;22:306. [Crossref] [PubMed]
- Weig T, Irlbeck M, Frey L, et al. Parameters associated with short- and midterm survival in bridging to lung transplantation with extracorporeal membrane oxygenation. Clin Transplant 2013;27:E563-70. [Crossref] [PubMed]
- Crotti S, Iotti GA, Lissoni A, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest 2013;144:1018-25. [Crossref] [PubMed]
- Oh DK, Hong SB, Shim TS, et al. Effects of the duration of bridge to lung transplantation with extracorporeal membrane oxygenation. PLoS One 2021;16:e0253520. [Crossref] [PubMed]
- Hayanga JW, Lira A, Aboagye JK, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg 2016;22:406-10. [Crossref] [PubMed]
- Zheng H, Yan D, Wang P, et al. Survival with Lung Transplantation and Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Minerva Respir Med 2022;61:6-15.
- Wan X, Bian T, Ye S, et al. Extracorporeal membrane oxygenation as a bridge vs. non-bridging for lung transplantation: A systematic review and meta-analysis. Clin Transplant 2021;35:e14157. [Crossref] [PubMed]
- Hayanga AJ, Aboagye J, Esper S, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: an evolving strategy in the management of rapidly advancing pulmonary disease. J Thorac Cardiovasc Surg 2015;149:291-6. [Crossref] [PubMed]
- Boling B, Dennis DR, Tribble TA, et al. Safety of Nurse-Led Ambulation for Patients on Venovenous Extracorporeal Membrane Oxygenation. Prog Transplant 2016;26:112-6. [Crossref] [PubMed]
- Habertheuer A, Richards T, Sertic F, et al. Stratification Risk Analysis in Bridging Patients to Lung Transplant on ECMO: The STABLE Risk Score. Ann Thorac Surg 2020;110:1175-84. [Crossref] [PubMed]
- Gottlieb J, Greer M. Recent advances in extracorporeal life support as a bridge to lung transplantation. Expert Rev Respir Med 2018;12:217-25. [Crossref] [PubMed]
- Du AL, Hayanga J, D’Cunha J, et al. Mechanical Ventilation and Extracorporeal Membrane Oxygenation (Ecmo) as a Combined Bridging Strategy to Lung Transplantation: Significant Gains in Survival. J Heart Lung Transplant 2017;36:S53. [Crossref] [PubMed]
- Chen A, Lian Q, Xu X, et al. Research Progress on Early Acute Kidney Injury after Lung Transplantation. Organ Transplantation 2020;11:743-8.
- Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 2012;7:1328-36. [Crossref] [PubMed]
- Speidl WS, Goliasch G, Roth C, et al. Association of Venoarterial Extracorporeal Membrane Oxygenation Cannulation Site with Weaning Time and Survival. J Am Coll Cardiol 2016;67:1431.
- Jiao G, Huang J, Wu B, et al. Association of Pulmonary Artery Pressure Change With Post-Lung Transplantation Survival: Retrospective Analysis of China Registry. JACC Asia 2022;2:819-28. [Crossref] [PubMed]
- Lüsebrink E, Stremmel C, Stark K, et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J Clin Med 2020;9:992. [Crossref] [PubMed]



