Pleurodesis with povidone-iodine, as an effective procedure in management of patients with malignant pleural effusion
Introduction
Malignant pleural effusion (MPE) arises in advanced-stages of malignancies and frequently heralds a poor prognosis (1). Most patients with MPE are symptomatic. The most common symptom is exertional dyspnea (2). Most patients undergo chemotherapy or local treatments to palliate symptoms such as dyspnea, cough & chest pain, to improve quality of life. If the underlying malignancy is chemo sensitive (e.g., small-cell carcinoma of lung & lymphoma), systemic chemotherapy may control the pleural effusion. However, when pleural effusion persists or reaccumulates after chemotherapy, the management of refractory MPE includes local therapeutic methods such as thoracentesis, pleurodesis, pleurectomy, or pleuroperitoneal shunting (3-5). Instilling of sclerosing agents into the pleural cavity (pleurodesis) is a common method for the management of MPE (5). For 70 years, many agents such as anti-neoplastics (e.g., nitrogen mustard, bleomycin), tetracycline derivatives, talc, erythromycin, silver nitrate, and povidone-iodine have been injected into the pleural cavity to create pleurodesis (6,7). The present prospective study aimed to investigate the efficacy and safety of pleurodesis with povidone-iodine (Betadine), as an inexpensive alternative agent for the local management of pleural effusion in patients with MPE admitted in Sari General Hospital during 2008-2011. Povidone-iodine is an antiseptic agent in different forms (topical solution, topical ointment, shampoo, surgical scrub) .In the current study, the authors use povidone-iodine in the form of 10% topical solution that contains 1% active iodine. Thus installation of this agent is associated with high iodine intake theoretically. Hence, the authors evaluate the effect of iodine on thyroid function by measuring the thyroid function tests before and after pleurodesis with povidone- iodine.
Methods
From October 2008 to June 2011, 42 patients were admitted to the Department of Thoracic Surgery because of symptomatic malignant pleural effusion. All patients had documentary malignant pleural effusion established by the positive result of pleural effusion cytology or pleural biopsy. Among these patients, three cases because of short life expectancy and three cases because of abnormal thyroid function test (n=1), history of previous pleurodesis with bleomycin (n=1), incomplete lung re-expansion after chest tube insertion (n=1) were excluded from the study. Also, all patients that participated in this study had normal renal function. A total of 36 patients were included in the study after each gave informed consent. The study was approved by the Ethics committee of Mazandaran University of Medical Sciences.
Because of the possibility of systemic absorption of iodine in povidone-iodine and severity of thyroid disease, thyroid function testing was done before the performance of procedure. Therefore, the patients with thyroid disease were excluded in the study. For the evaluation of effect of povidone-iodine on thyroid gland, the authors measured serum levels of TSH, T3 and T4 before and after pleurodesis at 1 week. Post-procedure abnormal thyroid function tests can be the indicators of role of iodine on thyroid gland, due to pleurodesis with povidone-iodine.
After local anesthesia with 1% injection solution of lidocaine (Caspian tamin, Rasht, Iran), a no. 28 thoracostomy tube (Supa, Tehran, Iran) was inserted into the pleural cavity through the fifth intercostals space in level of midaxillary line. Then, thoracostomy tube was connected to a water-seal drainage system to achieve complete drainage of effusion and lung re-expansion .When complete lung re-expansion was verified by lung CT scan, pleurodesis was performed at patient’s bedside. A mixture of 20 mL of 10% topical solution of povidone-iodine (Behvazan, Rasht, Iran) and 80 mL of normal saline (Daru pakhsh, Tehran, Iran) and 2 mg/kg lidocaine 2% (Caspian tamin, Rasht, Iran) was Instilled into the pleural cavity through thoracostomy tube and then, the tube was clamped for 2 hours. The position of these patients was changed within 2 hours by the medical staff to circulate the mixture. After declamping, the thoracostomy tube was removed as soon as the drainage decreased <100 mL per day. Negative pressure was not applied to any of the patients. After pleurodesis, all patients were assessed via chest X-ray (CXR) after 1 week, 1 and 3 months.
The response to this procedure, treatment failure and the complaints of the patients were evaluated. The authors defined “complete response” as symptomatic improvement of dyspnea with complete radiographic resolution of the pleural effusion and “partial response” as symptomatic improvement with recurrent pleural effusion that did not require additional thoracentesis and “treatment failure” as recurrent pleural effusion that required thoracentesis. All data were analyzed using SPSS (version 16.5). Chi-squares and paired-T test were used. P values <0.05 with CI=95% were considered statistically significant.
Results
Thirty six patients with refractory MPE were eligible to enter in the current study period. Four patients had bilateral MPE and pleurodesis was performed bilaterally, synchronously. The mean age of patients was 64.7±8.4 [49-80] years. Fifteen patients (41.6%) were men and 22 (58.3%) were women. The most common primary diseases were lung cancer (n=19, 52.7%) followed by breast cancer (n=8, 22.2%), ovarian cancer (n=5, 13.8%), gastric cancer (n=2, 5.5%) with an unknown origin (n=2, 5.5. The involved side in 21 patients (58.3%) was the right side, 11 patients (30.5%) was the left side and 4 patients (11.1%) was the bilateral. Twenty-six patients (72.2%) and 7 patients (19.4%) achieved confirmed complete and partial responses respectively, while 3 patients (8.3%) failed in treatment. The overall success rate was 91.6% (n=36). In post-procedure, the most common complaints of the patients were pain (n=14, 35.9%), followed by dyspnea (n=8, 20.5%), burning (n=7, 17.9%) and fever (n=3, 7.7%) (Table 1).
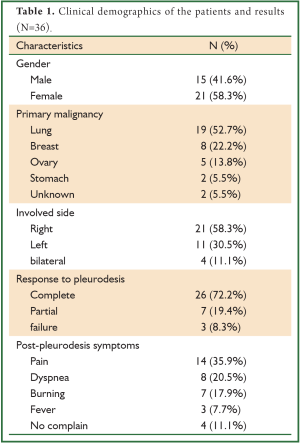
Full table
The recurrence of pleural effusion in control CXRs was not seen in 26 patients (72.2%).Recurrent effusion was mild in 7 patients (19.4) and severe in 3 patients (8.3%). The mean serum level of TSH was 1.9 and 2.6 µU/mL (P-value >0.05). Also, the average T4 was 7.4 and 7.7 µg/mL (P-value >0.05) and the average T3 was 1.34 and 1.35 µg/mL (P-value 0.05) respectively before and after pleurodesis.
Statistically, there was not significant relationship between response to treatment and some changes like the type of primary malignancy (P=0.683), patient gender (P=0.721), age (P=0.758), and the involved side (P=0.978). The statistical relation between post-pleurodesis complaints and the rate of response to treatment in patients were not significant (P=0.874). Before and after pleurodesis with povidone-iodine, the significant relationship in the quantities of serum levels of TSH (P=0.356, CI =95%), T4 (P=0.78, CI =95%) and T3 (P=0.425, CI =95%) was not found statistically. Also, there was not any mortality or morbidity (such as visual or neurological complications) due to the use of povidone-iodine in this study.
Discussion
Malignant pleural effusion (MPE) is found in malignant cells in pleural fluid or pleural tissue. MPE is a common complication of advanced-stages of malignancies (3-5,8). The current treatment options include repeated thoracocentesis through a thoracostomy tube or pleural catheter with or without chemical pleurodesis and pleuroscopy with pleurodesis. Chemicals pleurodesis is the best treatment for untreatable underlying disease (9).
In most patients with MPE, palliative treatment requires pleurodesis with sclerosing agents. The most cost-effective method for controlling of MPE is drainage through thoracostomy tube and intrapleural instillation of a chemical agent including talc, tetracycline (e.g., doxycycline), bleomycin and povidone-iodine (10). Talc is unavailable in IRAN and bleomycin, which more expensive with lower efficacy, is being used. Povidone-iodine is the most inexpensive among these agents, with an expense of less than 1€ per procedure. Talc, one of the most successful and widely used agents, has a success rate of >90% (11). However, its use has been associated with the following serious complications, including acute respiratory distress syndrome (ARDS), acute pneumonitis, systemic embolization and mortality (5-7,12,13).
In the current study, among 39 cases, 30 (76.9%) had the primary site of malignancy at lung and breast. This is generally attributed to the fact that carcinomas of breast and lungs are the most common malignancies to invade the pleura and produce MPE (4,14). Povidone-iodine (Betadine) is an iodine-based topical antiseptic agent, extensively absorbed from mucosal surfaces, leading to increase in serum iodine concentrations. It may be absorbed by the thyroid gland and may appear in saliva, sweat and milk, and is excreted unchanged in the urine. Although the exact mechanism by which povidone-iodine exerts its pleurodesis activity is unclear, it is thought to be related to the low pH of the sclerosing solution (pH =2.97) (7,15).
Olivares Torres et al. (7) performed pleurodesis with povidone-iodine in 52 cases and achieved control of the effusion in 96.1%. In their study, pleural effusion was malignant in 85% of the cases. Agarwal et al. (15) obtained complete response rate of 86.5% in pleural effusion group and 92.6% in pneumothorax group with povidone-iodine in a study including 37 patients with pleural effusion and 27 patients with pneumothorax. In a review of six studies, 265 patients underwent chemical pleurodesis with povidone-iodine, and the mean success rate was 90.6%. In this meta-analysis, pleurodesis with povidone-iodine was performed for recurrent pleural effusion in 157 patients and pneumothorax in 108 patients (16). Walker Renard et al. (17) reviewed all the published articles in the English language from 1966 to 1992 which described patients with recurrent symptomatic MPEs treated with chemical pleurodesis. Complete response was obtained in 60.4% of 1,168 patients. The reported complete response rate was highest for talc, at 93%, followed by 67% for tetracycline and 54% for bleomycin.
Agarwal et al. (16) reported that all the patients in their study experienced chest pain and noted that the only clinically important side effect of povidone-iodine was pain and chemical pleurodesis did not cause death in these patients. The other side effects reported were fever in seven patients and empyema in one patient. Olivares Torres et al. (7) detected serious chest pain and hypotension in three mesothelioma cases (5.8%) .
Although povidone-iodine is highly absorbed from mucosal surfaces and causes excess in serum levels of iodine and thereby may be associated with thyroid dysfunction, Yeginsu et al. (18) reported no changes in the serum levels of thyroid hormone at 24 and 72 hours after pleurodesis and in 8.7 month-follow-up period.
In the present study, complete response was obtained in 26 patients (72.2%) 7 patients with partial response (19.4%) and treatment failure in 3 patients (8.3%). The overall success rate was 91.6% (n=36). In post-procedure, the most common complaints of patients were pain (n=14, 35.9%) followed by dyspnea (n=8, 20.5%), burning (n=7, 17.9%) and fever (n=3, 7.7%). Although these complaints were not severe, but in these patients, control CXRs did not have any changes related to dyspnea after the instillation of povidone-iodine. Chest pain was improved by oral analgesic agents and dyspnea was eliminated with conservative managements (semi-setting positioning & O2 therapy) in short course. The recurrence of pleural effusion in control CXRs was not seen in 26 patients (72.2%).Recurrent effusion was mild in 7 patients (19.4%) and severe in 3 patients (8.3%). We also found no significant changes in thyroid function tests before and one week after pleurodesis with povidone-iodine.
Conclusions
In conclusion, povidone-iodine (Betadine) is an effective, inexpensive, safe, chemical pleurodesis feasible agents in the management of MPE.
Acknowledgements
With special gratitude to the Research Department of Mazandaran University of Medical Sciences for scientifically and financially supporting this study.
Disclosure: The authors declare no conflict of interest.
References
- Chen H, Brahmer J. Management of malignant pleural effusion. Curr Oncol Rep 2008;10:287-93.
- Stokes LS. Percutaneous management of malignant fluid collections. Semin Intervent Radiol 2007;24:398-408.
- Parulekar W, Di Primio G, Matzinger F, et al. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest 2001;120:19-25.
- Saffran L, Ost DE, Fein AM, et al. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest 2000;118:417-21.
- Sahn SA. Talc should be used for pleurodesis. Am J Respir Crit Care Med 2000;162:2023-4; discussion 2026.
- Dikensoy O, Zhu Z, Donnelly E, et al. Combination therapy with intrapleural doxycycline and talc in reduced doses is effective in producing pleurodesis in rabbits. Chest 2005;128:3735-42.
- Olivares-Torres CA, Laniado-Laborín R, Chávez-García C, et al. Iodopovidone pleurodesis for recurrent pleural effusions. Chest 2002;122:581-3.
- Tsai TH, Wu SG, Chang YL, et al. Effusion immunocytochemistry as an alternative approach for the selection of first-line targeted therapy in advanced lung adenocarcinoma. J Thorac Oncol 2012;7:993-1000.
- Kilic D, Akay H, Kavukçu S, et al. Management of recurrent malignant pleural effusion with chemical pleurodesis. Surg Today 2005;35:634-8.
- Muduly D, Deo S, Subi Ts, et al. An update in the management of malignant pleural effusion. Indian J Palliat Care 2011;17:98-103.
- Caglayan B, Torun E, Turan D, et al. Efficacy of iodopovidone pleurodesis and comparison of small-bore catheter versus large-bore chest tube. Ann Surg Oncol 2008;15:2594-9.
- Haas AR, Sterman DH, Musani AI. Malignant pleural effusions: management options with consideration of coding, billing, and a decision approach. Chest 2007;132:1036-41.
- Lee YC, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest 2003;124:2229-38.
- Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer 2006;54:1-9.
- Agarwal R, Aggarwal AN, Gupta D. Efficacy and safety of iodopovidone pleurodesis through tube thoracostomy. Respirology 2006;11:105-8.
- Agarwal R, Aggarwal AN, Gupta D, et al. Efficacy and safety of iodopovidone in chemical pleurodesis: a meta-analysis of observational studies. Respir Med 2006;100:2043-7.
- Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 1994;120:56-64.
- Yeginsu A, Karamustafaoglu A, Ozugurlu F, et al. Iodopovidone pleurodesis does not effect thyroid function in normal adults. Interact Cardiovasc Thorac Surg 2007;6:563-4.


