Primary pulmonary chondrosarcoma and a fast-growing mass that accidentally mimicked teratoma
Introduction
Primary pulmonary sarcoma is uncommon, constituting less than 1% of primary pulmonary malignancies (1). Primary pulmonary chondrosarcoma is even rarer. Only 39 English language publications describe cases of primary chondrosarcoma of the lung [5 cases of myxoid chondrosarcoma (2-5)]. Here, we report a rare case of hemoptysis with a fast-growing, invasive mass.
Case presentation
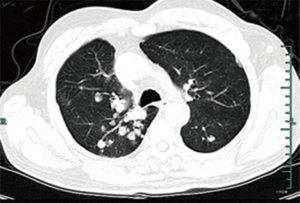
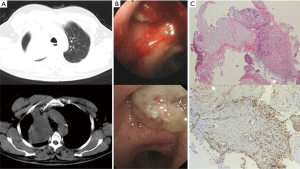
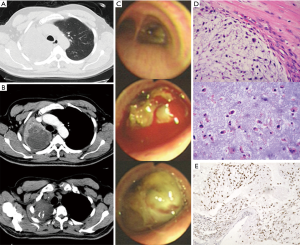
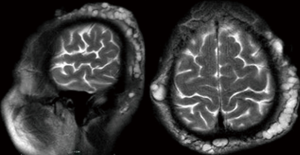
A 59-year-old male (former smoker) was admitted to the hospital after complaining of hemoptysis for 5 months and cough and dyspnea for 20 days. Five months before admission, a chest computed tomography scan (Figure 1A) revealed a mass in the upper lobe of the right lung (6 cm × 5 cm). The 99mTc bone scintigram and brain and abdomen computed tomography scans were negative. Bronchoscopy (Figure 1B) revealed a hemorrhagic neoplasm in the right upper bronchus. The biopsy specimen (Figure 1C) contained the following tissues: mucoid cartilage, fiber, respiratory epithelium and squamous epithelium. Immunohistochemical staining was positive for cytokeratin, vimentin, s-100, glial fibrillary acidic protein, smooth muscle actin, and epithelial membrane antigen; thus, we suspected that the mass was a benign or borderline tumor. The patient refused further operation. Five months later, the patient developed cough and worsening dyspnea. The complete blood count, erythrocyte sedimentation rate, C-reactive protein level, carcinoembryonic antigen level, alpha-fetoprotein level, human chorionic gonadotropin level, repeated bacteriological and cytological detections of the sputum, and tuberculosis skin test indicated no abnormalities. A chest computed tomography scan (Figure 2A) revealed a fast-growing mass in the upper lobe of the right lung (10.5 cm × 8 cm), and the enhancement was partially heterogeneous. The mass was inhomogeneous in density with calcification (25–43 Hu) (Figure 2B). The blood gas analysis revealed a pH of 7.44, PaCO2 of 38.7 mmHg, and PO2 of 67.4 mmHg. Bronchoscopy (Figure 2C) revealed a hemorrhagic mass obstructing the entrance to the right main bronchus. The lung function test indicated a mid-range, mixed ventilation disorder. Because malignancy was suspected, the patient underwent thoracotomy. According to frozen section pathology, the diagnosis during operation was myxoid chondrosarcoma with a negative margin. Therefore, right upper sleeve lobectomy with mediastinal lymphadenectomy was performed. Macroscopically, we observed a firm white mass 9.5 cm in diameter with a central area of necrosis. Histopathology (Figure 2D) revealed neoplastic chondrocytes with enlarged and hyperchromatic nuclei in the myxoid matrix. The tumors were positive for S-100 (Figure 2E) and negative for cytokeratin, epithelial membrane antigen, smooth muscle actin, vimentin, glial fibrillary acidic protein, desmin, CD34, thyroid transcription factor-1, chromogranin A, and synaptophysin. The Ki-67 labelling index was 20%. Lymph node dissection revealed no evidence of metastasis. Eight months after operation, a chest computed tomography scan revealed multiple pulmonary nodules (Figure 3), and magnetic resonance imaging of the skull revealed subcutaneous skull metastases (Figure 4) (suggesting recurrence). The patient is currently undergoing adriamycin and ifosfamide chemotherapy.
Discussion
Chondrosarcoma of the lung usually metastasizes from skeletal and extra-skeletal tumors. The present patient had no history of skeletal or soft tissue tumors. These tumors probably originated from the bronchial cartilage. Extra-osseous chondrosarcoma can be divided into five histologic varieties: myxoid, mesenchymal, dedifferentiated, hyaline and highly differentiated. Primary pulmonary chondrosarcoma is also categorized into tracheobronchial and lung varieties. In the 5 patients with primary myxoid chondrosarcomas of the lung reported in literatures, patients range from 35 to 69 years old. It appeared a male predominance. There is no prevalence regarding tumor location. Patients mainly complain of fever, cough, dyspnea, and chest pain. The tumors are up to 11 cm in diameter. The most important clinical features of the five patients are depicted in Table 1. Immunohistochemically, chondrosarcoma is positive for S-100. In the present patient, S-100-positive cells were located in the chondromatous component. Pulmonary teratoma, hamartoma, chondroma, osteosarcoma, carcinosarcoma, epithelioid hemangioendothelioma and signet ring cell carcinoma are considered for differential diagnosis. Teratomas are composed of two or three germ layers. Malignant teratomas are accompanied by elevated serum alpha-fetoprotein or human chorionic gonadotropin levels. In the present patient, the bronchoscopy biopsy specimen was composed of mucoid cartilage, fiber, respiratory epithelium and squamous epithelium which deriving from endoderm and ectoderm tissues. It mimicked the appearance of a teratoma. This might be due to the biopsy needle puncture position has a deviation and it punctured the tissue surrounding the mass. Hamartomas are generally composed of cartilage, fat and connective tissue cells with cleft-like spaces lined by low columnar or cuboidal epithelium. Hamartomas may show some chondroid atypia but there is far less atypia than chondrosarcoma. Pulmonary chondroma is usually associated with gastric stromal sarcoma and extra adrenal paraganglioma. Pulmonary chondromas are composed of myxoid cartilage and wide areas of ossification. Osteosarcomas are composed of atypical osteoid tissue. Carcinosarcoma commonly shows a carcinomatous component or epithelial differentiation. Epithelioid hemangioendotheliomas are characterized by small, diffuse nodules in both lungs (via computed tomography scans), and stain positive for factor VIII or CD31. Signet ring cell carcinoma stains positive for keratin antibodies, and the nuclei are pushed to the periphery. Until now, there has been no standard therapy for primary pulmonary chondrosarcoma. Most patients have been treated with extensive surgical resection. A few patients underwent effective radiation or chemotherapy. The tumor is chemosensitive to adriamycin and ifosfamide (6). It was reported that interferon alfa-2b was useful for the treatment of this disease (4). The tumor can metastasize to the skeleton, skin and kidney. Death is usually caused by intrathoracic spread. This type of tumor usually grows slowly, metastasizes late, and responds well to excision when localized. The longest survival of a patient with primary pulmonary myxoid chondrosarcoma was 6 years after operation (3).

Full table
This patient presented with tracheobronchial myxoid chondrosarcoma, and we have intact imaging, bronchoscopic and pathologic records. The tumor progressed rapidly within 5 months (from 6 to 10 cm in diameter), and the patient relapsed 8 months after operation. The tumor grows slowly in the initial symptom-free phase when localized. Then, a symptomatic phase ensues, during which the tumor progresses rapidly. This tumor type presents as unique subcutaneous metastases. Due to malignancy, adjuvant chemotherapy or radiation is required. The bronchoscopy biopsy specimen was composed of two germ layers and mimicked teratoma because of puncture location deviation. This finding implies that a definite diagnosis requires gross specimen detection, and a biopsy sample may not always make sense.
Acknowledgements
Funding: This work was supported by research grants from the Zhejiang Provincial Natural Science Foundation of China [LY15H010003], the Major Project of the Science Technology Department of Zhejiang Province [2012C13022-2] and Project of Health and Family Planning Commission of Zhejiang Province, China [2013KYB119].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Huang HY, Hsieh MJ, Chen WJ, et al. Primary mesenchymal chondrosarcoma of the lung. Ann Thorac Surg 2002;73:1960-2. [Crossref] [PubMed]
- Kalhor N, Suster S, Moran CA. Primary pulmonary chondrosarcomas: a clinicopathologic study of 4 cases. Hum Pathol 2011;42:1629-34. [Crossref] [PubMed]
- Ichimura H, Endo K, Ishikawa S, et al. Primary chondrosarcoma of the lung recognized as a long-standing solitary nodule prior to resection. Jpn J Thorac Cardiovasc Surg 2005;53:106-8. [Crossref] [PubMed]
- Rubinger M, Plenderleith IH, Lertzman M, et al. Metastatic extraskeletal myxoid chondrosarcoma. Successful therapy with interferon alfa-2b. Chest 1995;108:281-2. [Crossref] [PubMed]
- Watanabe A, Ito M, Nomura F, et al. Primary chondrosarcoma of the lung--a case report with immunohistochemical study. Jpn J Med 1990;29:616-9. [Crossref] [PubMed]
- Patel SR, Vadhan-Raj S, Burgess MA, et al. Results of two consecutive trials of dose-intensive chemotherapy with doxorubicin and ifosfamide in patients with sarcomas. Am J Clin Oncol 1998;21:317-21. [Crossref] [PubMed]