
Effect of OK-432 pleurodesis on long-term survival outcomes after pulmonary lobectomy for lung cancer patients: a retrospective case-control study
Highlight box
Key findings
• OK-432 pleurodesis effectively manages postoperative complications such as air leaks and chylothorax in patients undergoing pulmonary lobectomy for non-small cell lung cancer.
• No significant improvements were observed in overall survival, lung cancer-specific survival, or recurrence-free survival when compared to a control group.
What is known and what is new?
• OK-432 is recognized for its efficacy in managing postoperative air leaks and for its immunomodulatory properties in various cancers.
• This study confirms the effectiveness of OK-432 pleurodesis in post-surgical management but does not support improved long-term survival outcomes, contrasting with its known effects in other cancer treatments.
What is the implication, and what should change now?
• The findings indicate that while OK-432 pleurodesis effectively manages air leaks and chylothorax after lung cancer surgery, it does not improve long-term survival outcomes. This highlights a need to reassess the role of OK-432 in enhancing survival in such cases. Future research should focus on larger, multicenter retrospective studies to thoroughly investigate OK-432’s immunomodulatory potential and optimize its clinical use. These studies are crucial for examining whether adjustments in timing or dosage can enhance survival outcomes, which would guide its strategic application in thoracic oncology.
Introduction
OK-432 (Picibanil, Chugai Pharmaceutical Co., Tokyo, Japan), a preparation of Streptococcus pyogenes, type A3, serves as an immunotherapeutic agent against various cancers (1), originally developed by Okamoto et al. (2). Furthermore, its application extends to pleurodesis for the management of malignant pleural effusions associated with diverse cancer types, including primary lung cancer (3-7), underscoring its versatile therapeutic potential. Moreover, OK-432 has been recognized as an effective agent for chemical pleurodesis in patients with spontaneous pneumothorax (8-10). Its application is also notable for addressing prolonged postoperative air leaks following thoracic surgery (11,12). Although not covered by health insurance in Japan, OK-432 is frequently used clinically for spontaneous pneumothorax and postoperative air leaks. While it is associated with increased inflammatory reactions compared to talc, its efficacy and safety for pleurodesis remain comparable (12).
In our clinical practice, OK-432 is primarily used for addressing prolonged air leaks or chylothorax after pulmonary resections in lung cancer patients. This study was initiated based on the hypothesis that OK-432 could offer secondary benefits beyond its immediate therapeutic role, potentially enhancing long-term outcomes for lung cancer patients undergoing surgery. The dual potential of OK-432 as both a pleurodesis agent and an immunotherapeutic suggests a need for a deeper exploration of its broader impacts, particularly in improving survival outcomes post-surgery. Therefore, this research aims to investigate the effects of intrapleural OK-432 administration on long-term survival and recurrence among lung cancer patients following pulmonary lobectomy, providing critical insights into its potential benefits beyond conventional pleurodesis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2253/rc).
Methods
Patients, data collection, and study groups
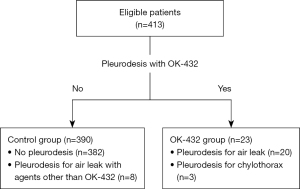
We performed a retrospective analysis on data from 441 patients who underwent pulmonary lobectomy for non-small cell lung cancer at Tohoku University Hospital from January 2010 to December 2016. Twenty-eight patients were excluded from the study due to various reasons: insufficient data (n=8), neoadjuvant treatment prior to surgery (n=14), pleural dissemination observed during surgery (n=3), and incomplete resection (n=3). The analysis ultimately included 413 patients, divided into two study groups: the control group, which included patients who either did not undergo pleurodesis or underwent pleurodesis with agents other than OK-432 for prolonged postoperative air leaks or chylothorax, and the OK-432 group, which consisted of patients treated with OK-432 pleurodesis (Figure 1). We gathered information on preoperative demographics, surgical and pathological details, incidence and management of postoperative air leaks and pleurodesis, as well as other postoperative complications. Postoperative complications were classified according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 (13) or the Clavien-Dindo classification system (14,15). Complications of grade 2 or higher in the CTCAE or grade II or higher in the Clavien-Dindo classification system were regarded as significant complications.
Postoperative surveillance consisted of monthly outpatient visits during the first two months, followed by visits every 3–6 months over a period of 5 years. Recurrence of the disease was monitored through clinical examination, computed tomography (CT), positron emission tomography/CT (PET/CT), or magnetic resonance imaging (MRI). The date of recurrence was established as the date of diagnosis judged by imaging or histological evidence. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The institutional review board (IRB) of Tohoku University Graduate School of Medicine approved this retrospective study (No. 2021-1-912-1) and waived the requirement for informed consent.
OK-432 pleurodesis for prolonged air leak or chylothorax after pulmonary lobectomy
Pulmonary lobectomies were conducted on patients with non-small cell lung cancer either through open-chest surgery or video-assisted thoracic surgery (VATS). At the conclusion of the procedure, a chest tube was inserted via a port incision and directed toward the apex of the thoracic cavity. The chest tube was removed by postoperative day 3 if there was no air leak or chylous pleural effusion. When OK-432 pleurodesis was indicated due to prolonged air leak or chylothorax, 5 to 10 KE of OK-432 dissolved in 100 mL saline was injected into the thoracic cavity through the chest tube. After the injection, to ensure the solution remained in the thoracic cavity, we clamped the tube for one hour before resuming low-pressure continuous suction. If clamping was not feasible due to severe air leaks, we instead applied water-seal suction for one hour. We monitored air leaks for 2 to 3 days post-pleurodesis, performing additional OK-432 pleurodesis when necessary. In cases of chylothorax, we monitored the amount of pleural effusion for 4 to 5 days, performing additional pleurodesis as required. While pleurodesis was primarily performed using OK-432 to manage prolonged air leaks or chylothorax, alternative agents such as minomycin and a 50% glucose solution were utilized for some patients, considering potential systemic inflammatory reactions to OK-432.
Statistical analysis
Data are expressed as means with ranges or as numbers (%). Student’s t-test or Welch’s t-test was used for parametric data. Mann-Whitney U test was used for nonparametric data. Fisher’s exact test or the Chi-squared test was performed for categorical values. Survival rates—overall survival (OS), lung cancer-specific survival (LCSS), and recurrence-free survival (RFS)—were calculated using the Kaplan-Meier method, with differences evaluated via the log-rank test. Mortality and recurrence risk factors were analyzed using univariate and multivariate Cox proportional hazards models, presenting hazard ratios with 95% confidence intervals. Statistical analyses were conducted using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) and JMP Pro 17 (SAS Institute Inc., Cary, NC, USA). Values of P<0.05 were regarded as significant.
Results
Pleurodesis and study groups
Of the 413 patients analyzed, 23 required OK-432 pleurodesis for managing prolonged postoperative air leaks (20 patients) or chylothorax (3 patients), categorized as the OK-432 group. These patients received either one or two intrapleural doses of OK-432, which effectively managed the air leaks and chylothorax. Specifically, two patients needed OK-432 pleurodesis twice. The remaining 390 patients, forming the control group, either did not undergo pleurodesis (382 patients) or received pleurodesis with agents other than OK-432 (8 patients) for prolonged postoperative air leaks (Figure 1).
Preoperative demographics
As shown in Table 1, there were no significant differences between the groups in age, gender, height, body mass index, smoking history, or performance status. Preoperative pulmonary function tests also showed no significant differences.
Table 1
| Variables | Control group (N=390) | OK-432 group (N=23) | P value |
|---|---|---|---|
| Age (years) | 68.9±0.4 [36–86] | 67.5±1.8 [53–83] | 0.43 |
| Gender | 0.20 | ||
| Male | 216 (55.4) | 16 (69.6) | |
| Female | 174 (44.6) | 7 (30.4) | |
| Height (cm) | 160.4±0.4 [134–183] | 160.3±2.1 [142–184] | 0.96 |
| Body weight (kg) | 59.6±0.5 [38.0–97.8] | 58.0±2.7 [37.4–89.0] | 0.47 |
| BMI (kg/m2) | 23.1±0.2 [15.6–36.3] | 22.5±0.9 [13.8–25.4] | 0.37 |
| Smoking history | 0.11 | ||
| Former and current smoker | 256 (65.6) | 11 (47.8) | |
| Never | 134 (34.4) | 12 (52.2) | |
| Performance status | >0.99 | ||
| 0 | 353 (90.5) | 21 (91.3) | |
| 1 and 2 | 37 (9.5) | 2 (8.7) | |
| Past medical history | – | ||
| Hypertension | 80 (20.5) | 4 (17.4) | |
| Ischemic heart disease | 38 (9.7) | 2 (8.7) | |
| Diabetes mellitus | 37 (9.5) | 2 (8.7) | |
| Malignant disease | 32 (8.2) | 2 (8.7) | |
| Cerebrovascular disease | 21 (5.4) | 0 (0) | |
| Interstitial lung disease | 15 (3.8) | 0 (0) | |
| Arrhythmia | 11 (2.8) | 0 (0) | |
| Preoperative pulmonary function test | |||
| % predicted FVC | 108.2±0.8 [62.4–169.2] | 108.1±3.5 [80.4–153.1] | 0.99 |
| % predicted FEV1 | 105.5±1.0 [57.4–158.8] | 98.3±2.7 [70.8–124.6] | 0.07 |
| % predicted DLco | 105.8±1.2 [52.3–185.7] | 102.5±4.0 [65.1–149.5] | 0.51 |
| Clinical stage | – | ||
| 0 | 9 (2.3) | 0 (0) | |
| IA | 195 (50.0) | 12 (52.2) | |
| IB | 73 (18.7) | 4 (17.4) | |
| II | 80 (20.5) | 3 (13.0) | |
| III | 33 (8.5) | 4 (17.4) | |
| Operative time (min) | 223±4.7 [30–665] | 256±16.4 [53–83] | 0.09 |
| Blood loss (mL) | 92±9.0 [1–1,704] | 79±14.8 [1–255] | 0.74 |
| Lobectomy procedure types | 0.84 | ||
| RUL | 127 (32.6) | 9 (39.1) | |
| RML | 21 (5.4) | 1 (4.3) | |
| RLL | 68 (17.4) | 4 (17.4) | |
| Bilobectomy (RUML or RMLL) | 13 (3.3) | 1 (4.3) | |
| LUL | 83 (21.3) | 6 (26.1) | |
| LLL | 78 (20.0) | 2 (8.7) | |
| Postoperative complications | |||
| Pulmonary complications† | 80 (20.5) | 5 (21.7) | 0.89 |
| Cardiovascular complications | 34 (8.7) | 1 (4.3) | 0.46 |
| Histologic type | 0.86 | ||
| Adenocarcinoma | 284 (72.8) | 17 (73.9) | |
| Squamous cell carcinoma | 81 (20.8) | 4 (17.4) | |
| Others | 25 (6.4) | 2 (8.7) | |
| Pathological stage | – | ||
| 0 | 1 (0.3) | 0 (0) | |
| IA | 212 (54.4) | 14 (60.9) | |
| IB | 64 (16.4) | 2 (8.7) | |
| II | 69 (17.7) | 1 (4.3) | |
| III | 44 (11.3) | 6 (26.1) | |
| Postoperative adjuvant chemotherapy | – | ||
| Platinum-based chemotherapy | 45 (11.5) | 3 (13.0) | |
| Tegafur-uracil (oral) | 26 (6.7) | 0 (0) | |
| No | 319 (81.8) | 20 (87.0) | |
Data are expressed as group mean ± standard error [range] or number (%). †, postoperative air leaks were not included. BMI, body mass index; DLco, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LLL, left lower lobectomy; LUL, left upper lobectomy; RLL, right lower lobectomy; RML, right middle lobectomy; RMLL, right middle and lower lobectomy; RUL, right upper lobectomy; RUML, right upper and middle lobectomy.
Surgical characteristics and pathological stage
Table 1 shows the details of the surgical characteristics, showing no significant differences in operative times, blood loss, or types of lobectomy performed. Histologic types were similarly distributed across groups. However, comparisons of pathological staging were not feasible due to the insufficient number of patients at stage 0 in the OK-432 group.
Postoperative survival and recurrence rates
Figure 2A-2C show no significant differences in OS, LCSS, and RFS between groups. Figure 3A,3B indicate a non-significant trend towards lower local recurrence rates in the OK-432 group.


Risk factors for OS
Analysis from the univariate Cox proportional hazards model (Table 2) identifies smoking history, each preoperative pulmonary function, non-adenocarcinoma histologic type, advanced pathological stage (II or III), and postoperative pulmonary complications as significant predictors for OS. Analysis from the multivariate Cox proportional hazards model (Table 3) identifies smoking history, preoperative pulmonary function [% predicted forced vital capacity (FVC)], advanced pathological stage (II or III), and postoperative pulmonary complications as significant predictors for OS. OK-432 pleurodesis showed no impact on OS in both univariate and multivariate analyses.
Table 2
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 0.995 | 0.974–1.019 | 0.69 |
| Gender (male/female) | 1.438 | 0.930–2.224 | 0.10 |
| BMI | 0.966 | 0.905–1.029 | 0.29 |
| Smoking history (yes/no†) | 2.912 | 1.692–5.014 | <0.001 |
| Preoperative pulmonary function test | |||
| % predicted FVC | 0.974 | 0.961–0.987 | <0.001 |
| % predicted FEV1 | 0.988 | 0.977–1.011 | 0.04 |
| % predicted DLco | 0.990 | 0.980–1.010 | 0.046 |
| Histologic type (non-AD/AD) | 2.330 | 1.523–3.567 | <0.001 |
| Pathological stage (II or III/0 or I) | 3.259 | 2.137–4.970 | <0.001 |
| Postoperative pulmonary complication (yes/no) | 2.638 | 1.698–4.097 | <0.001 |
| Postoperative cardiovascular complication (yes/no) | 0.477 | 0.175–1.302 | 0.11 |
| Postoperative platinum-based chemotherapy (yes/no) | 1.648 | 0.955–2.844 | 0.09 |
| Pleurodesis (OK-432/control‡) | 1.617 | 0.780–3.354 | 0.23 |
†, yes/no, former and current smoker/never; ‡, control, no pleurodesis or pleurodesis with agents other than OK-432. AD, adenocarcinoma; BMI, body mass index; CI, confidence interval; DLco, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Table 3
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 0.996 | 0.973–1.022 | 0.75 |
| Gender (male/female) | 1.451 | 0.920–2.288 | 0.10 |
| BMI | 0.971 | 0.914–1.029 | 0.44 |
| Smoking history (yes/no†) | 2.076 | 1.141– 3.779 | 0.02 |
| Preoperative pulmonary function test | |||
| % predicted FVC | 0.981 | 0.967–0.995 | 0.007 |
| % predicted FEV1 | 1.004 | 0.991–1.019 | 0.49 |
| % predicted DLco | 0.998 | 0.987–1.010 | 0.78 |
| Histologic type (non-AD/AD) | 0.872 | 0.513–1.481 | 0.61 |
| Pathological stage (II or III/0 or I) | 3.599 | 2.176–5.950 | <0.001 |
| Postoperative pulmonary complication (yes/no) | 2.357 | 1.459–3.808 | <0.001 |
| Postoperative cardiovascular complication (yes/no) | 0.456 | 0.164–1.271 | 0.09 |
| Postoperative platinum-based chemotherapy (yes/no) | 1.613 | 0.856–3.037 | 0.13 |
| Pleurodesis (OK-432/control‡) | 1.752 | 0.819–3.747 | 0.18 |
†, yes/no, former and current smoker/never; ‡, control, no pleurodesis or pleurodesis with agents other than OK-432. AD, adenocarcinoma; BMI, body mass index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLco, carbon monoxide diffusing capacity.
Risk factors for RFS
Analysis from the univariate Cox proportional hazards model (Table 4) identifies non-adenocarcinoma histologic type, advanced pathological stage (II or III), postoperative pulmonary complications, and postoperative platinum-based chemotherapy as significant predictors of RFS. The multivariate Cox proportional hazards model (Table 5) identifies only advanced pathological stage (II or III) as a significant predictor of RFS. OK-432 pleurodesis showed no impact on RFS in either the univariate or multivariate analyses.
Table 4
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 0.999 | 0.979–1.020 | 0.89 |
| Gender (male/female) | 1.074 | 0.732–1.574 | 0.72 |
| BMI | 1.036 | 0.977–1.097 | 0.24 |
| Smoking history (yes/no†) | 1.287 | 0.857–1.934 | 0.22 |
| Preoperative pulmonary function test | |||
| % predicted FVC | 0.990 | 0.979–1.002 | 0.10 |
| % predicted FEV1 | 0.992 | 0.982–1.002 | 0.13 |
| % predicted DLco | 0.993 | 0.985–1.002 | 0.14 |
| Histologic type (non-AD/AD) | 1.717 | 1.158–2.547 | 0.007 |
| Pathological stage (II or III/0 or I) | 3.838 | 2.620–5.622 | <0.001 |
| Postoperative pulmonary complication (yes/no) | 1.731 | 1.129–2.652 | 0.01 |
| Postoperative cardiovascular complication (yes/no) | 0.917 | 0.463–1.816 | 0.81 |
| Postoperative platinum-based chemotherapy (yes/no) | 2.785 | 1.777–4.364 | <0.001 |
| Pleurodesis (OK-432/control‡) | 0.983 | 0.431–2.240 | 0.97 |
†, yes/no, former and current smoker/never; ‡, control, no pleurodesis or pleurodesis with agents other than OK-432. AD, adenocarcinoma; BMI, body mass index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLco, carbon monoxide diffusing capacity.
Table 5
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 0.997 | 0.977–1.020 | 0.91 |
| Gender (male/female) | 1.008 | 0.681–1.490 | 0.93 |
| BMI | 1.031 | 0.972–1.092 | 0.33 |
| Smoking history (yes/no†) | 0.776 | 0.480– 1.253 | 0.29 |
| Preoperative pulmonary function test | |||
| % predicted FVC | 0.995 | 0.982–1.008 | 0.47 |
| % predicted FEV1 | 0.998 | 0.986–1.011 | 0.76 |
| % predicted DLco | 0.999 | 0.989–1.001 | 0.82 |
| Histologic type (non-AD/AD) | 1.157 | 0.711–1.882 | 0.54 |
| Pathological stage (II or III/0 or I) | 3.717 | 2.486–5.557 | <0.001 |
| Postoperative pulmonary complication (yes/no) | 1.541 | 0.950–2.382 | 0.08 |
| Postoperative cardiovascular complication (yes/no) | 0.870 | 0.433–1.746 | 0.76 |
| Postoperative platinum-based chemotherapy (yes/no) | 0.997 | 0.856–3.037 | 0.55 |
| Pleurodesis (OK-432/control‡) | 0.965 | 0.415–2.243 | 0.92 |
†, yes/no, former and current smoker/never; ‡, control, no pleurodesis or pleurodesis with agents other than OK-432. AD, adenocarcinoma; BMI, body mass index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLco, carbon monoxide diffusing capacity.
Discussion
This study investigated the anticancer effects of one or two intrapleural doses of OK-432, used as a pleurodesis agent, for improving OS, LCSS, and RFS following pulmonary lobectomy in patients with non-small cell lung cancer. Although OK-432 effectively managed postoperative complications such as air leaks and chylothorax, we did not observe significant improvements in OS, LCSS, or RFS when compared to the control group.
Despite the known role of OK-432 in eliciting immune responses in cancer treatment, the limited immune activation observed with the dosages administered in our study suggests that higher or more frequent dosing might be necessary to achieve meaningful clinical benefits. Additionally, the timing of administration relative to surgical intervention could critically influence its efficacy.
Previous studies have demonstrated OK-432’s immunomodulatory benefits in other cancer contexts. For instance, in a study by Kikkawa et al., OK-432 was used as adjuvant immunotherapy in patients with cervical carcinoma. Intradermal administration of OK-432 commenced postoperatively with an initial dosage of 1 KE three times a week, gradually increasing to 5 KE, was maintained at this level bi-weekly for 2 years. This study revealed that, while there was no significant difference in RFS between the groups, patients in stage II without lymph node metastasis showed a significantly higher RFS in the OK-432 group, suggesting a potential benefit of OK-432 for enhancing the immune responsiveness in specific patient subgroups (16). Similarly, Tano et al. investigated the effects of OK-432 in combination with oral tegafur-uracil and radiotherapy in patients with oral squamous cell carcinoma (17). OK-432 was administered intradermally at a dose of 0.5 KE once a week for five weeks as part of their immunochemoradiotherapy protocol. They found that patients who received the combination of radiotherapy, tegafur-uracil, and OK-432 exhibited significantly longer OS and progression-free survival compared to those who received only radiation therapy (RT) and tegafur-uracil. The study further explored the mechanisms underlying these effects, demonstrating that OK-432, when combined with 5-fluorouracil (5-FU) and X-ray irradiation, enhances the induced helper T cell 1 (Th1) response. This enhancement was mediated by the inhibition of regulatory T cells and Th2 cytokines through the modulation of specific transcription factors and signaling pathways, revealing a synergistic effect of immunochemoradiotherapy (17). However, our study, although effective at managing postoperative air leaks and chylothorax, did not significantly improve OS, LCSS, or RFS. This may be due to the limited immune activation by only one or two doses of OK-432, or the timing of its administration, which might not be optimal for eliciting a robust immunotherapeutic response.
The retrospective design and the relatively small sample size, particularly among patients undergoing OK-432 pleurodesis, significantly constrain our capacity to definitively ascertain its efficacy as an immunotherapeutic agent. A notable limitation of the current study is the comparison of outcomes among patients treated with different pleurodesis agents, including OK-432. Regrettably, the cohort receiving alternative pleurodesis agents was too small to allow for a robust comparative analysis. While prospective studies would be the gold standard for such investigations, they are currently not feasible due to the unique clinical applications of OK-432.
Given these constraints, future research should focus on retrospective analyses involving larger patient cohorts across multiple centers. To enhance the validity of these studies, employing propensity score matching will be crucial to adjust for potential confounders and ensure comparability between treatment groups. This approach will enable a more comprehensive assessment of OK-432’s therapeutic effectiveness and permit exploration of the impacts of timing and dosage adjustments on clinical outcomes. By expanding the scope and scale of the research, employing advanced statistical methods, and overcoming the current study’s limitations, we aim to provide clearer insights into the potential benefits of OK-432 in clinical practice.
Conclusions
In summary, while OK-432 pleurodesis effectively manages specific postoperative complications such as air leaks and chylothorax in lung cancer surgery as previously reported, its influence on long-term survival in lung cancer patients appears limited under the conditions explored in this study. Further retrospective research, involving larger patient cohorts and detailed data analysis, is essential to fully explore the immunomodulatory capabilities of OK-432 and its optimal application in the management of lung cancer post-surgery.
Acknowledgments
We thank Mr. Brent Bell, an independent English editor, for his assistance with editing this manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2253/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2253/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2253/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2253/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The institutional review board (IRB) of Tohoku University Graduate School of Medicine approved this retrospective study (No. 2021-1-912-1) and waived the requirement for informed consent. Instead, the need for consent was addressed through an opt-out mechanism, as per institutional guidelines. Patients were informed about the study through public disclosure on the institutional website, allowing them the opportunity to decline participation. The IRB approval was granted by the Tohoku University Graduate School of Medicine, which oversees research ethics at Tohoku University Hospital, including the Department of Thoracic Surgery, Institute of Development, Aging and Cancer, Tohoku University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hashimoto M, Takashige K, Furuyashiki M, et al. Enhancement of antitumor activity of OK-432 (picibanil) by Triton X-114 phase partitioning. Int Immunopharmacol 2008;8:12-9. [Crossref] [PubMed]
- Okamoto H, Shoin S, Koshimura S, et al. Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol 1967;11:323-6. [Crossref] [PubMed]
- Kishi K, Homma S, Sakamoto S, et al. Efficacious pleurodesis with OK-432 and doxorubicin against malignant pleural effusions. Eur Respir J 2004;24:263-6. [Crossref] [PubMed]
- Ishida A, Miyazawa T, Miyazu Y, et al. Intrapleural cisplatin and OK432 therapy for malignant pleural effusion caused by non-small cell lung cancer. Respirology 2006;11:90-7. [Crossref] [PubMed]
- Kasahara K, Shibata K, Shintani H, et al. Randomized phase II trial of OK-432 in patients with malignant pleural effusion due to non-small cell lung cancer. Anticancer Res 2006;26:1495-9. [PubMed]
- Yoshida K, Sugiura T, Takifuji N, et al. Randomized phase II trial of three intrapleural therapy regimens for the management of malignant pleural effusion in previously untreated non-small cell lung cancer: JCOG 9515. Lung Cancer 2007;58:362-8. [Crossref] [PubMed]
- Nohara K, Takada K, Kojima E, et al. A propensity score-matched comparison of the efficacies of OK-432 and talc slurry for pleurodesis for malignant pleural effusion induced by lung adenocarcinoma. Respir Investig 2016;54:341-6. [Crossref] [PubMed]
- How CH, Hsu HH, Chen JS. Chemical pleurodesis for spontaneous pneumothorax. J Formos Med Assoc 2013;112:749-55. [Crossref] [PubMed]
- Ogawa K, Takahashi Y, Murase K, et al. OK-432 pleurodesis for the treatment of pneumothorax in patients with interstitial pneumonia. Respir Investig 2018;56:410-7. [Crossref] [PubMed]
- Shinno Y, Kage H, Chino H, et al. Old age and underlying interstitial abnormalities are risk factors for development of ARDS after pleurodesis using limited amount of large particle size talc. Respirology 2018;23:55-9. [Crossref] [PubMed]
- How CH, Tsai TM, Kuo SW, et al. Chemical pleurodesis for prolonged postoperative air leak in primary spontaneous pneumothorax. J Formos Med Assoc 2014;113:284-90. [Crossref] [PubMed]
- Watanabe T, Yamauchi Y, Takeyama R, et al. A Comparison of the Efficacies of OK-432 and Talc Slurry for Pleurodesis in Patients with Prolonged Air Leak after Pulmonary Resection. Ann Thorac Cardiovasc Surg 2024;30:23-00115. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. [Accessed 18 May 2024] [Internet]. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kikkawa F, Kawai M, Oguchi H, et al. Randomised study of immunotherapy with OK-432 in uterine cervical carcinoma. Eur J Cancer 1993;29A:1542-6. [Crossref] [PubMed]
- Tano T, Okamoto M, Kan S, et al. Immunochemoradiotherapy for patients with oral squamous cell carcinoma: augmentation of OK-432-induced helper T cell 1 response by 5-FU and X-ray irradiation. Neoplasia 2013;15:805-14. [Crossref] [PubMed]


