
Efficacy of Amplatzer device for endobronchial treatment of hemoptysis: case series
Highlight box
Key findings
• This study demonstrates that endobronchial deployment of the AmplatzerTM Vascular Plug device (AVPD) achieved hemostasis in 93% of patients with massive or sub-massive hemoptysis, with minimal recurrence post-removal.
What is known and what is new?
• AVPD has been demonstrated to be an effective treatment for the closure of bronchopulmonary fistulas.
• This is the first case series on using AVPD for hemoptysis, demonstrating its effectiveness as a definitive or bridging treatment.
What is the implication, and what should change now?
• The AVPD can be efficiently used in bronchoscopy for addressing hemoptysis, either as a stand-alone treatment or as a bridging measure to a definitive procedure.
• The use of AVPD in hemoptysis may improve bleeding control during bronchoscopy and reduce the need for post-procedure surgery.
Introduction
Hemoptysis, expectoration of blood from the lower respiratory tract, can range in severity from mild and self-limiting to massive and life-threatening. The etiology of hemoptysis is varied, attributable primarily to bronchiectasis, lung neoplasms and respiratory infections, most commonly Mycobacterium tuberculosis in endemic developing countries (1,2).
The treatment of hemoptysis varies depending upon its severity. While mild to moderate bleeding can be managed by antibiotics and/or tranexamic acid, patients with significant hemoptysis may require urgent evaluation to identify the bleeding source by contrast-enhanced computed tomography chest angiography (CTA) or bronchoscopy. When bleeding persists despite conservative measures, endovascular, endobronchial or surgical interventions are warranted to achieve hemostasis (3,4).
Bronchoscopy allows for localization of the bleeding source, and the implementation of endobronchial treatments to achieve hemostasis, ranging from endoscopic installation of vasoconstrictors (e.g., adrenalin and cold saline) or tranexamic acid, targeted treatment of a visible bleeding vessel with local coagulation therapy (e.g., argon plasma laser or electrocautery), and the endobronchial isolation of the bleeding source by wedge tamponade. Various bronchial blockers have been deployed to this end, including Fogarty balloons, silicon spigots, endobronchial stents, and endobronchial valves (5).
The AmplatzerTM Vascular Plug device (AVPD, Abbott Medical, St. Paul, MN, USA), designed for vascular embolization in the peripheral vasculature, has been repurposed for endobronchial applications, principally occluding endobronchial fistulas (6-9). The AVPD provides various advantages as a bronchial blocker, including ease of deployment and removal, self-expanding design and proven safety profile of inert nitinol metal. The utilization of AVPD for hemoptysis management has been rarely described (10). We present a case series of our experience with endobronchial deployment of AVPD for hemoptysis management. We present this article in accordance with the STROBE and AME Case Series reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1783/rc).
Methods
We conducted a case series retrospective analysis of adult (≥18 years old) patients who underwent bronchoscopy for hemoptysis at our academic Rabin Medical Center (Petah Tikva, Israel), a tertiary university hospital, during the period of March 2018–May 2024, and identified all patients where an endobronchial AVPD was deployed for hemoptysis management. We collected data from the electronic database including demographics, medical history, hemoptysis etiology, bronchoscopy findings, subsequent management and patient outcomes.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Rabin Medical Center (RMC-0284-24). Patient consent was waived by our Institutional Review Board due to the retrospective design of this study, with all data de-identified and analyzed per ethical standards.
Massive hemoptysis was defined either quantitatively by an estimated volume of ≥100 mL/h or ≥150 mL/24 h, or qualitatively if it caused hemodynamic or respiratory instability. Hemodynamic instability was defined as either hypotension (systolic blood pressure <90 mmHg or diastolic <60 mmHg) or the requirement of blood products and/or vasoconstrictor agents to stabilize blood pressure. Respiratory instability was defined as low oxygen saturation [peripheral saturation <92% or decrease in >5% from patient’s baseline value (11)] or the initiation of invasive/non-invasive ventilation. Patients who had stopped smoking for at least six months before the hemoptysis event were considered former smokers.
For each variable analyzed, the data was obtained from review of the relevant patient’s electronic record in the hospital’s electronic record database. As this was a retrospective study, we attempted to address bias by including all patients in the electronic database who met the inclusion criteria.
Statistical analysis
Descriptive statistics were employed for analysis, presenting continuous variables via median, range, interquartile range (IQR), and categorical variables in terms of frequency and percentage.
Results
During the study period, 15 patients (9 men, 6 women) presenting with hemoptysis were managed by endobronchial deployment of an AVPD to achieve hemostasis (Figure 1). Patient demographics, medical history, hemoptysis etiology, clinical characteristics, bronchoscopy procedure, outcomes and follow-up are summarized in Table 1. Patient median age was 60 years (range, 24–86 years), and 11 (73%) patients were former or current smokers. Eleven patients (73%) presented with massive hemoptysis, four with sub-massive hemoptysis. Four patients (27%) were receiving anti-platelet treatment, none were receiving anticoagulants. Blood test results revealed no potentially reversible coagulopathy at the time of presentation.
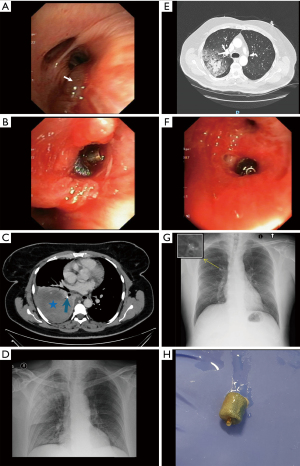
Table 1
| Patient No. | Sex | Age (years) | Smoking status (PY) | Massive hemoptysis | Clinical status | Emergency procedure | Treatment before bronchoscopy | Procedure after bronchoscopy | Bleeding site | AVPD size (mm) | AVPD out (days) | Cause | Follow-up duration (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | Active, 100 | Yes | Stable | No | INH TXA | – | RUL | 12 | †* | Unknown | 5 |
| 2 | F | 48 | Former, 10 | Yes | Hem | Yes | INH TXA, BAE | Surgery | RML, lateral | 8 | ‡ | Bronchiectasis | 1,191 |
| 3 | M | 41 | Active, 40 | Yes | Hem, Resp | No | INH TXA | – | LUL, lingula | 8 | * | Surgical stump | 2 |
| 4 | M | 78 | Former, 35 | No | Resp | No | INH TXA, BAE | – | RUL, posterior | 10 | * | Unknown | 3 |
| 5 | F | 44 | No | NA | Stable | No | INH TXA | – | RLL, superior | 4 | NA | Benign mass | 671 |
| 6 | F | 48 | No | No | Stable | No | – | – | LUL | 10 | – | Bronchiectasis | 600 |
| 7 | F | 57 | Former, NA | No | Stable | No | INH TXA | – | LUL, lingula | 6 | 391 | Surgical stump | 509 |
| 8 | M | 36 | Active, 10 | Yes | Stable | Yes | – | – | RLL, medial | 6 | 44 | ANCA vasculitis | 467 |
| 9 | M | 62 | Former, 100 | Yes | Hem, Resp | Yes | INH TXA, BAE | Surgery | LUL, anterior | 10 | ‡ | Iatrogenic | 29 |
| 10 | M | 86 | Active, 45 | Yes | Stable | No | INH TXA | – | LLL, posterior | 6 | 8 | Unknown | 461 |
| 11 | M | 60 | Active, 40 | Yes | Stable | No | INH TXA | BAE | RUL, anterior | 8 | 4 | Bronchiectasis | 170 |
| 12 | F | 65 | Active, 68 | Yes | Stable | No | – | Surgery | RUL, apical | 6 | ‡ | NSCLC | 129 |
| 13 | M | 62 | No | Yes | Stable | Yes | INH TXA | – | RUL, posterior | 6 | 23 | ANCA vasculitis | 60 |
| 14 | M | 24 | No | Yes | Stable | No | INH TXA | – | RUL | 8 | – | Unknown | 25 |
| 15 | M | 84 | Former, 50 | Yes | Stable | No | INH TXA | – | RLL, medial | 6 | – | Unknown | 5 |
Patients are arranged in chronological order of their presentation. †, the Amplatzer treatment failed due to size mismatch; ‡, the patient required surgical treatment; *, the patient died within a week after bronchoscopy. ANCA, antineutrophil cytoplasmic antibody; AVPD, AmplatzerTM Vascular Plug device; BAE, bronchial artery embolization; F, female; Hem, hemodynamic instability; INH, inhalation route; LLL, left lower lobe; LUL, left upper lobe; M, male; NA, data not available; NSCLC, non-small cell lung cancer; PY, pack-years; Resp, respiratory instability; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; TXA, tranexamic acid.
Etiology of hemoptysis was not identified for 5 patients (33%), infected bronchiectasis for 3 patients (20%), bleeding from surgical stump for 2 patients (13%), antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides for 2 patients (13%), and one patient with each of lung cancer, a space-occupying lesion with benign pathology, and iatrogenic bleeding after bronchoscopic thermal vapor ablation.
All but one of the patients underwent computed tomography (CT) chest angiography for hemoptysis evaluation and localization prior to the bronchoscopy. Three patients (20%) underwent bronchial artery embolization before bronchoscopic intervention. Four patients (27%) required emergent bronchoscopy performed during on-call hours.
In all cases the location of bleeding was identified during bronchoscopy. Thirteen patients (87%) were treated with tranexamic acid either by inhalation before bronchoscopy (12 patients) and/or by endobronchial installation or intravenous infusion during bronchoscopy (6 patients). The most common used AVPD diameter was 6 mm (6 cases).
Hemostasis was achieved through endobronchial AVPD deployment in 14 (93%) patients. AVPD deployment was unsuccessful in the remaining patient because the diameter of the identified bronchus exceeded the diameter size of the AVPD available at the time of the procedure in the bronchoscopy suite.
Patients were followed for a median of 129 days post bronchoscopy (Table 1). The majority of patients (60%) were followed for more than two months, with six being followed for more than one year. The shortest follow up periods corresponded to the three patients who experienced mortality within a week after bronchoscopy and one patient that underwent procedure 5 days before the data base was accessed. Following AVPD deployment, all surviving patients underwent follow-up bronchoscopy within one month post-deployment. During follow-up bronchoscopy, the AVPD was removed in five patients, as hemoptysis had resolved, the underlying cause of bleeding had been adequately treated, and the risk of recurrence was deemed low. Subsequent patient follow-up, whether in an outpatient clinic or for repeat bronchoscopy, varied based on clinical judgment and the severity of underlying disease.
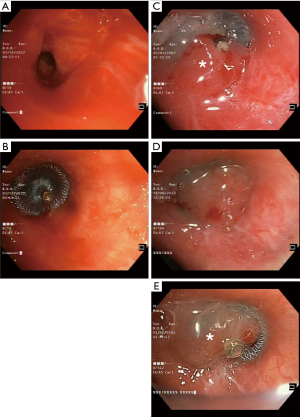
No procedure-related complications were observed during the follow-up period. Four patients required subsequent surgery or embolization due to bleeding recurrence after a median of 12 days. Eight patients achieved durable hemostasis; in five patients, the AVPD was removed after a median period of 23 days without bleeding recurrence, and in three patients, the AVPD remains deployed in place (Figure 2). The remaining three patients expired within one week of the bronchoscopy for causes unrelated to hemoptysis or the AVPD deployment.
Discussion
Massive hemoptysis is a potentially life-threatening condition, which requires prompt evaluation and treatment. First line treatment for massive or sub-massive hemoptysis is bronchial artery embolization when active bleeding is identified on CTA. Bronchoscopy plays an important role in emergent procedures or when embolization is unavailable or impracticable. Bronchoscopy allows for localization of the bleeding site and achieving hemostasis by various endobronchial techniques, ranging from bronchoscopic installation of vasoconstrictors or tranexamic acid, local coagulation therapy (e.g., argon plasma laser or electrocautery), and the endobronchial isolation of the bleeding source by wedge tamponade. Various bronchial blockers have been deployed to this end, including Fogarty balloons, silicon spigots, endobronchial stents, and endobronchial valves (5).
AVPD is a medical device primarily used in the field of interventional radiology for the treatment and embolization of vascular abnormalities in the peripheral vasculature. In the field of bronchoscopy, AVPD has been demonstrated to be a safe and effective treatment for closure of bronchopulmonary fistulas, due to its flexible design and range of sizes (7-9). The properties of AVPD similarly make it a suitable device for the endobronchial management of hemoptysis.
AVPD is a cylindrical plug made of a self-expanding inert nitinol mesh, with a proven safety profile, both in endovascular and endobronchial use (Figure 1H). Upon deployment during bronchoscopy, the device expands to occlude the bronchus and thereby isolates the bleeding source (Figure 1, Videos 1,2). These properties allow the AVPD to accommodate different airway diameters and shapes and minimize the risk of device migration. The nitinol alloy is a radiopaque material, that can be visualized on chest X-rays and CT images (Figure 1, Video 2), allowing for post-procedure surveillance.
Bronchoscopy allows for simultaneous direct videoscope visualization of the bronchial source of bleeding and targeted deployment of the AVPD under fluoroscopy guidance. AVPD are available in ranges of 4 to 16 mm, which enables selection of the diameter that most appropriately conforms to the target bronchus. In our study the selection of an appropriately sized AVPD was performed through visual estimation of the bronchial orifice diameter during bronchoscopy. As a general rule, we typically use devices with a diameter of 14–16 mm for the main bronchus, 10–12 mm for lobar bronchi, and 6–8 mm for segmental airways. It is important to note that the chosen AVPD size should slightly exceed the airway diameter to ensure a secure fit and minimize the risk of post-deployment migration, given the device’s flexibility and self-expanding properties.
AVPD can be repositioned prior to full deployment under fluoroscopy guidance, enabling accurate positioning and isolation of the bleeding source. The AVPD can either be maintained in situ as a definitive treatment of hemoptysis or subsequently removed after the risk of subsequent hemoptysis has subsided.
In this study, we demonstrate the efficacy of the AVPD for treatment of massive and sub-massive hemoptysis secondary to various etiologies. AVPD deployment as an endobronchial blocker at the bleeding site effectively attains immediate hemostasis, and was deployed successfully both as a definitive treatment for hemoptysis, as well as a temporary stop-gap measure to bridge to subsequent surgery or bronchial artery embolization. While one procedure did not achieve hemostasis due to a mismatch between the AVPD diameter and the bronchus size, it was the first procedure performed in our center, prior to the availability in our bronchoscopy suite of greater diameter AVPDs which can accommodate larger bronchi.
The utilization of AVPD in hemoptysis management has been rarely described, limited to our knowledge to a case report involving massive hemoptysis, where the AVPD was deployed via rigid bronchoscopy (10). This study is the first case series encompassing a heterogeneous cohort of adult patients, including cases of both massive and sub-massive hemoptysis. Notably, all interventions in our series were performed using a flexible bronchoscope under conscious sedation and were well tolerated, with no reported adverse events or procedural complications.
Our study has several limitations. This is a retrospective study and may have a recall bias. Moreover, this is a single-center study with a small cohort that might not be reproducible in a larger multi-center study.
Conclusions
In this study, we present our experience in achieving durable hemostasis with endobronchial deployment of the AVPD for massive and sub-massive hemoptysis. AVPD deployment into the bleeding airway is an effective and safe technique, and was deployed successfully both as a definitive treatment for hemoptysis, as well as a bridge to definitive surgery or embolization. The current study, to our knowledge, is the first case series describing the successful deployment of AVPD for the treatment of hemoptysis. Further research in a larger patient population, including comparison of results with standard of care treatment for hemoptysis is required to further substantiate our results and the efficacy of AVPD in hemoptysis management.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE and AME Case Series reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1783/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1783/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1783/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1783/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Rabin Medical Center (RMC-0284-24). Patient consent was waived by our Institutional Review Board due to the retrospective design of this study, with all data de-identified and analyzed per ethical standards.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mondoni M, Carlucci P, Job S, et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur Respir J 2018;51:1701813. [Crossref] [PubMed]
- Singh SK, Tiwari KK. Etiology of hemoptysis: A retrospective study from a tertiary care hospital from northern Madhya Pradesh, India. Indian J Tuberc 2016;63:44-7. [Crossref] [PubMed]
- Tsai YS, Hsu LW, Wu MS, et al. Effects of Tranexamic Acid on Hemoptysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig 2020;40:789-97. [Crossref] [PubMed]
- O'Gurek D, Choi HYJ. Hemoptysis: Evaluation and Management. Am Fam Physician 2022;105:144-51. [PubMed]
- Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010;80:38-58. [Crossref] [PubMed]
- Kramer MR, Peled N, Shitrit D, et al. Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Chest 2008;133:1481-4. [Crossref] [PubMed]
- Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest 2011;139:682-7. [Crossref] [PubMed]
- Gershman E, Azem K, Heesen P, et al. Amplatzer Occluders for Effective Nonsurgical Management of Bronchopleural Fistulae. Ann Thorac Surg 2024;118:225-32. [Crossref] [PubMed]
- Freidkin L, Azem K, Pertzov B, et al. Endobronchial closure of broncho-biliary fistula using Amplatzer device: Case report. Respir Med Case Rep 2023;46:101943. [Crossref] [PubMed]
- Mahajan AK, Mahajan AK, Thompson J, et al. The Novel Use of the Amplatzer Occlusion Device to Aid in the Treatment of Massive Hemoptysis. Surg Case Reports. 2020;1-3.
- Beasley R, Chien J, Douglas J, Eastlake L, Farah C, King G, et al. Target oxygen saturation range: 92–96% Versus 94–98%. Respirology 2017;22:200-2. [Crossref] [PubMed]


