
New standardized five-zone lobectomy with structured assessment in robotic surgery: the French lobectomy
Highlight box
Surgical highlights
• Standardization of robotic lobectomies can improve teaching and learning the technique.
• It can also improve surgical performance reducing operating room times and costs associated to robotic lobectomies.
What are the conventional and novel/modified techniques?
• This technique is based in a five exposure and dissection zones different than what has been described in a step-by-step approach.
• This gives freedom to the surgeon to complete the lobectomy at his/her will.
• We propose an objective evaluation scale to assess the trainee progress.
What are the implications, and what should change now?
• The standardized approach could be useful in teaching robot-assisted thoracic surgery (RATS) to new robotic surgeons.
• The objective evaluation scale could be useful in proposing recommendations to the trainees.
• This could open a new methodology to certify RATS surgeons, other than industry certification, in an academic based training.
Introduction
Background
Robotic surgery is a minimally invasive technique that was first introduced in Europe in 1999. In the year 2000, the Da Vinci robot (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was approved by the Food and Drug Administration (FDA) (1). Today, over 6,000 robotic platforms are in use around the world with more than 10 million procedures already performed.
Nowadays, minimal invasive approaches as video-assisted thoracic surgery (VATS) and robot-assisted thoracic surgery (RATS) are the gold standard (2) for pulmonary resections, especially in the early stages of lung cancer and there is an increasing use of robotic surgery worldwide (3). The introduction of VATS supposed a new approach to lung resections with vessel division performed first and the massification of fissure less technique to complete resection. Our RATS technique allows to come back to the approach performed earlier for many years by thoracotomy with a fissure base approach.
Rational
It can, however, be difficult for surgeons, to gain sufficient access to the robotic console, resulting in intermittent exposure of surgeons to RATS. Moreover, teaching a new technique from start is challenging, especially given the fact that for lobar resection already, five new procedures need to be mastered. RATS learning curve may be slow and technical skills differ from VATS and open surgery (4). A standardised technique is therefore crucial to optimize safety, surgical quality, good oncological outcomes and to lower operating times.
To date, no academic certified RATS training program is available and “certification” is currently industry based.
Standardising the robotic curriculum, as well as robotic procedures, could reduce the learning curve. Confounding variables associated with new platforms and previous VATS experience could be diminished as well (5,6).
Deconstructing robotic lobectomy to teach RATS has been described. This could involve up to nineteen technical steps to complete a lobectomy (7). However, to date, no assessment scale has been proposed. Further methodologic refinements and studies are needed to evaluate teaching and a surgeon’s progress, but objective evaluation criteria are lacking. In our academic training centres in France, we have developed a standardized robotic thoracic approach for lobectomy and have implemented this training approach successfully over recent years in boot camps, masterclasses, fellows and in the French and European RATS curriculum.
Objectives
Our objective is to describe our standardized approach to RATS lobectomies and propose an objective structured assessment scale to evaluate progress and quality of surgery of new trainees. We share our experience with both the approach as well as the assessment scale. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1755/rc).
Preoperative preparations and requirements
In 2020, the thoracic surgery team of Rouen University Hospital implemented a standardized technique to perform robotic lobectomy. The technique was based on five exposure and dissection zones. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. As this is a report of our surgical technique, no Institutional Review Board (IRB) was considered necessary by the ethics committee of our institution. Written informed consent was obtained from every patient who has been operated on with this technique and for publication of the accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Careful patient selection is done including lobectomies, anatomic segmentectomies and difficult approaches of posterior nodules for non-anatomic resection and sulcus tumours. A careful analysis of the anatomy is made on computed tomography (CT) scans, as well as three-dimensional (3D) reconstruction images, assessing potential risks during RATS.
The surgical team is composed by a console surgeon, a resident beside the operation table and a scrubbing nurse. Anaesthesiologist is assisted by a trained nurse.
In robotic thoracic surgery several additional features have to be taken into consideration. This includes trocar positioning, lung manipulation and five-zone exposure, tissue dissection and energy use, lymphadenectomy technique, surgical strategy, perioperative and intraoperative communication with table-surgeon, scrub nurse and anaesthesiologist team. Intraoperative communication relies only on the voice.
Trocar positioning
The technique is based on four robotic arms and an assistant port. We use Air Seal® (ConMed Corporation, Largo, FL, USA) for humified CO2 insufflation. Pressure is set at 5 mmHg and raised to 10 mmHg if needed and if tolerated by the patient.
Trocars are configured in a W shape (Figure 1). Trocar diameter is based on the type of pulmonary resection done. The diameter of the camera trocar and the posterior trocar is 8 mm and the diameter of the anterior trocar is 12 mm. The diameter of the other trocars, between the camera and the posterior arms, is based on the type of pulmonary resection done: 8 or 12 mm (Figure 1).
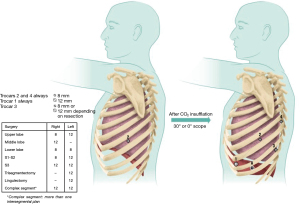
The positioning of trocars is also based on the type of resection done. We install the camera trocar between the 8th intercostal space for upper lobes and 9th intercostal space for middle and lower lobes but this can be modified to a more cranial or caudal intercostal space depending on patient size (small patients we use the 9th intercostal space). The W shape described above is done in conformity with the first trocar placement.
Trocar placement is essential to avoid future instrumentation conflicts. Port positions are systematically drawn on the skin.
Lung manipulation and five-zone exposure
Gentle movements, rotating more than pulling, are used to avoid lung damage and bleeding, extending operative time and prolonged air leak after surgery (Videos 1,2). Rotation movements are used to expose the hilum inferiorly, posteriorly, superiorly, and anteriorly. This allows a standardized approach into five exposure zones (Figures 2,3) and to enhance the mobilization of the lung, by opening all the mediastinal pleural and fissure for a better comprehension of the anatomy. Nevertheless, according to anatomical implication and the indicated resection, the dissection can be more extensive in the targeted zone (e.g., Zone 1, 2 and 5 for lower lobes).
Dissection instruments and energy use
Right-handed surgeons used a Maryland grasper in their right hand and a fenestrated bipolar grasper in their left hand, and vice versa for left-handed surgeons. These graspers are placed on the robotic arms situated adjacent to the camera port. Instruments are powered with bipolar coagulation energy. A tip-up grasper is used on the posterior arm. These instruments allow an excellent exposure with economic movements when exposing the dissection zones.
Two or three gauze rolls are introduced through the assistant port. These gauzes allowed exposure movements without parenchymal injury and can be used for compression if bleeding occurs.
Lymphadenectomy technique
The goal of lymphadenectomy is to expose and collect lymph nodes, but this is also pivotal in dissecting the bronchial and vascular structures and to prepare each structure to be divided after completing lymphadenectomy.
Each lymph node group is completely removed, without grabbing the lymph node to avoid tearing and bleeding.
Surgical strategy and fissure-based technique
Once exposure and dissection are completed, the broncho-vascular structures are divided. The order in which the structures were divided is dependent on the surgeon’s preference and the anatomical variations of the patient. In our practice, more than 95% of cases are done with a fissure-based technique, exposing the artery in the fissure and subsequently dividing the vessel.
Perioperative communication
Isolation of the surgeon in the robotic console highly impact the communication with other members of the surgical and anaesthesiology team. This could rise the anxiety of the group leading to stress and miscommunication (8). A pre-operative briefing is done in order to rise a proper team situational awareness and avoid potential threats during surgery depending on human factors. This needs to be taken into consideration.
Intraoperative monitoring is the same than for other thoracic surgeries. A clear visualisation from the console to the anaesthetist is secured in order to have a good communication between him and the surgeon in case of need.
Step-by-step description
Right lobectomy
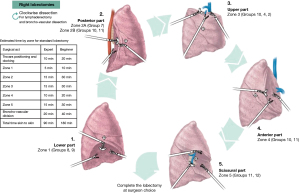
Exposure and dissection are done clockwise starting at the pulmonary ligament and continuing to the posterior hilum (Figure 2).
Zone 1
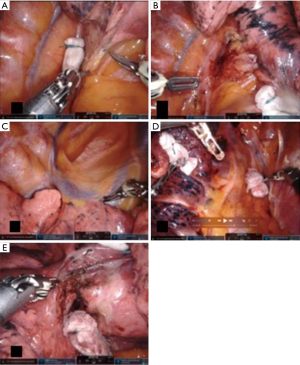
Zone one is defined as the inferior part of the hilum, mainly the pulmonary ligament. Exposure of the pulmonary ligament is done by anterior rotation and then gently pushing the inferior lobe superiorly with gauze rolls, which allowed a good exposure of the ligament. Dissection is done by separating the ligament up to the lower border of the inferior pulmonary vein. Lymph node dissection involved Naruke stations 8 and 9 (Figure 4A).
Zone 2
Zone 2 is defined as the posterior part of the hilum from the lower border of the inferior pulmonary vein up to the azygous vein arch. Exposure is done by anterior rotation of the inferior S6 segment. Dissection of the mediastinal pleura exposed the main bronchus and subcarinal region. Lymph node dissection was done posteriorly in Naruke stations 7 and anteriorly, in the inter-bronchial group 11, often referred to as the “sump” node. The underlying pulmonary artery at that level is managed with care. Removing these lymph nodes secures a path from anterior to posterior when dissecting the fissure later in the procedure. The superior border of the inferior pulmonary vein is dissected to prepare the passage of a vessel loop posteriorly in a lower lobectomy (Figure 4B).
Zone 3
Zone 3 is defined as the superior part of the hilum. Exposure is done by gently pulling the upper lobe down with a gauze roll. Lymph node dissection involves Naruke stations 10, 4 and 2. Dissection starts posteriorly at the upper lobe bronchus, continuing anteriorly with the pulmonary artery and finishes with the division (but not dissection) of the pulmonary artery and the superior pulmonary vein (Figure 4C).
Zone 4
Zone 4 is defined as the anterior part of the hilum. Exposure is done by gently rotating the lung posteriorly with gauze rolls. Pulmonary veins are exposed. Lymph node dissection is done anteriorly in Naruke stations 10 and 11. This is important to complete the anterior part of the fissure if necessary. Dissection of anterior veins is prepared in case they needed to be divided later (Figure 4D).
Zone 5
Zone 5 is defined as the fissure. Exposure is done by gently retracting the upper lobe superiorly with the tip up grasper. This is the only time the lung can be grabbed directly. The pulmonary arteries of interest are dissected. Lymph node dissection involved Naruke stations 11 and 12. Dissection over the artery creating a tunnel between the parenchyma and the artery is done towards posterior to complete the posterior fissure (Figure 4E). The division of the fissure can be done either with stapling device or energy depending on its thickness and the possibility of developing a prolonged air leak. Division of the different segmental arteries is done subsequently. After division of the arteries, the bronchus can be identified. Finally, the vein and bronchus can be divided using a robotic stapler. The remaining fissures can be completed thereafter. For upper lobes veins can be divided with the parenchyma with no need to dissect them separately.
Left lobectomy
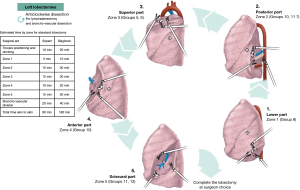
Exposure and dissection are done counter-clockwise starting at the pulmonary ligament and continuing to the posterior hilum (Figure 3).
Zone 1
Zone 1 exposure is done in the same way as for the right lobectomy. Lymph node dissection involves Naruke station 9 (Figure 5A).

Zone 2
Exposure is done by anterior rotation of the inferior S6 segment. Dissection of the mediastinal pleura exposes the left main bronchus and Naruke lymph node station 7. Lymph node dissection is done anteriorly for Naruke stations 10 and 11. The superior border of the inferior pulmonary vein is dissected to prepare the passage of a vessel loop posteriorly in the resection of the lower lobe (Figure 5B).
Zone 3
Zone 3 exposure is done in the same way as for the right lobectomy. The pulmonary artery is dissected up to the superior pulmonary vein. Lymph node dissection involves Naruke stations 10, 5 and 6. Dissection finishes with the division of the pulmonary artery and the superior pulmonary vein (Figure 5C).
Zone 4
Exposure is done by gently rotating the lung posteriorly with roll gauzes. Pulmonary veins are exposed. Lymph node dissection is done anteriorly for Naruke stations 10 and 11. This is important to complete the anterior part of the fissure if necessary. Dissection of anterior veins is prepared in case they needed to be divided later (Figure 5D).
Zone 5
Zone 5 exposure is done in the same way as for the right lobectomy, using the tip up grasper and opening the pleura in the fissure. Subsequently, the pulmonary artery is identified and dissected. Lymph node dissection involves Naruke stations 11 and 12. Dissection over the artery, creating a tunnel between the artery and parenchyma is done posteriorly to enable completion of the posterior fissure. Dissection and division of the different segmental arteries is done thereafter (Figure 5E). Finally, after division of the arteries, the pulmonary vein can be divided, followed by the bronchus using robotic staplers. The remaining fissures can be completed subsequently.
After completing the anatomical resection, the specimen is removed in a surgical bag through the assistant port. A drain is placed in the anterior robotic trocar. All trocars, except the camera trocar, are removed. Lung expansion is controlled under vision. After re-ventilation, and extraction of the specimen, the robot can be de-docked and surgery is finished.
Postoperative consideration and tasks
Postoperative care following robotic thoracic surgery is similar to other approaches in thoracic surgery as described in guidelines for enhanced recovery after lung surgery, focussing on pain management, early mobilization and early drain removal (9).
Usual follow up is done in the same manner than for other thoracic surgeries.
Objective and structured assessment
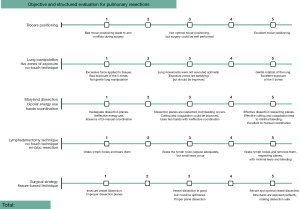
In parallel, we use an objective structured assessment scale to evaluate the performance of surgeons in training who use the standardized surgical technique. The scale is based on five items: trocar positioning; lung manipulation and five-zone exposure; dissection, instruments and bipolar energy use and hand coordination; lymphadenectomy technique; and surgical strategy. Each item is scored from 0 to 5 points with a maximum score of 25 points for the sum of all five items (Figure 6). A score of less than 15 points is indicative for a need for additional simulation training and focus on trocar positioning. A score between 15 and 20 points indicated to need for proctoring by a fully trained robotic thoracic surgeon. Surgeons with a score of more than 20 points are considered to be proficient.
Tips and pearls
The standardized surgical robotic approach for a lobectomy provides a framework to break down a multi-step operation into distinct components. The uniformity of this standardization makes the method applicable to a variety of thoracic surgeries on both the left and right side, facilitating teaching robotic thoracic surgery. By using a standardized assessment form, the surgeon’s learning curve can be quickly and consistently evaluated.
Discussion
Surgical highlights
In this work, we describe new objective criteria for robotic lobectomy, including a standardized surgical technique based on five exposure and dissection zones, and an objective structured assessment scale.
A standardised technique based on exposure zones allows to dissect different parts of the hilum in a very organized manner. One important point of this standardisation is that lymphadenectomy is done by zone including complete N1 and N2 lymph node resection which is the gold standard in current lung cancer surgery (10).
Freedom to choose which vascular structure to divide first is also an advantage of our standardised technique. As lymph nodes are dissected, bronchial and vascular structures become are liberated and dividing them is easier. Surgeons are free to choose when and in which order to dissect these structures. In our experience this is more flexible and implies less risk of a major vascular injury. Flexibility also includes that if a vessel is easier to be divided first, this could be done in order to remove lymph nodes in a safer way.
Strengths and limitations
The surgeon’s isolation in the console is a major concern when teaching robotic surgery, modifying the traditional master/apprentice relationship in surgery (11). However, a standardized technique based on exposure zones allows surgery to be more easily directed. Teachers may allow trainees to perform a range of procedures, from less complex procedures (e.g., exposure only) to more complex ones (e.g., lymph node dissection and vascular dissection) as trainees gain dexterity. A validation of this statement as well as the assessment scale is needed. This paper is the first phase of a research project including time operation, effects on the learning curve as well as the feedback from the trainees.
Comparison with other surgical techniques and researches
Some authors have proposed deconstructing robotic lobectomy, in a step-by-step approach (7,12). The issue with these approaches is that pulmonary anatomy is highly variable, especially in segmental anatomy. Another concern is that this “strict” form of teaching lobectomy leaves very little margin for surgeons faced with other forms of pulmonary resection and that every surgical procedure needs its own step-by-step approach.
Assessment of technical skills is very important. Objective structured assessment of technical skills (OSATS) was validated in 1997 to evaluate operative tasks in surgical residents and recently a modification of this evaluation was validated on robotic tasks for dry laboratories (dry labs) (13). The Global Evaluative Assessment of Robotic Skills (GEARS) rating system is a useful tool to evaluate robotic surgical skills in prostatectomy. GEARS grades proficiency in six domains: depth perception, bimanual dexterity, efficiency, force sensitivity, autonomy, robotic control (14). This scale could be applied to other surgeries because it evaluates general aspects of robotic surgery.
To our knowledge, no structured evaluation has been developed for thoracic surgery. We propose an objective structured assessment scale specifically for pulmonary resection based on 5 items.
Implications and actions recommended
Teaching RATS is challenging, as learning on a console implies isolation, which alters the traditional master/apprentice relationship in surgery (11). Robotic platforms have some advantages though. Dry labs realistic models allow to mimic real procedures, and to acquire advanced skills, leading to reduced training costs (15). Double consoles allow more traditional training, as the trainer is able to guide the trainee intra-operatively and regain control of surgery if needed (16).
Different platforms and instruments could be a confusing factor when teaching RATS (15). The impact of using different platforms and teaching RATS should be evaluated in future researches.
Perioperative management is important to obtain better surgical outcomes (17). Adding robotic surgery to an enhanced recovery program has been shown to decrease complications and hospital length of stay (18). Standardising procedures is also beneficial. Repeating gestures and automatizing them lead to shorter operative times (13). Shorter anaesthesia times contribute to the post-operative rehabilitation of patients especially those with poor lung function.
One essential point in robotic surgery is good communication in the operative room. Poor communication leads to bad results (19). Standardising the surgical technique and nomenclature during the procedure improves communication and has a beneficial effect on non-technical skills, which enhances the surgical team behaviour. This means working in a safer environment decreasing team anxiety and potential risks to patients (20).
Further research on assessing a robotic trainee improvement based on a standardised technique is needed and we hope to validate our scale soon to be useful to others while teaching and learning RATS lobectomies.
Conclusions
We propose a standardised surgical technique based on five exposure and dissection zones and an objective structured assessment scale that could improve surgeons’ performance and training in robotic lobectomy. This new technique allows both training in robotic lobectomy and assessment of the skills acquired.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs, CHU Rouen, for her help with editing the manuscript.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1755/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1755/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1755/coif). J.M.B. is proctor for Intuitive and consultant for Medtronic and Johnson and Johnson. I.B. is consultant for Intuitive, BD, Medela, MSD and Medtronic. P.B.P. is consultant for intuitive. F.M. has received fees from Intuitive. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. As this is a report of our surgical technique, no Institutional Review Board (IRB) was considered necessary by the ethics committee of our institution. Written informed consent was obtained from every patient who has been operated on with this technique and for publication of the accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shah J, Vyas A, Vyas D. The History of Robotics in Surgical Specialties. Am J Robot Surg 2014;1:12-20. [Crossref] [PubMed]
- Sihoe ADL. Video-assisted thoracoscopic surgery as the gold standard for lung cancer surgery. Respirology 2020;25:49-60. [Crossref] [PubMed]
- Terra RM, Leite PHC, Dela Vega AJM. Global status of the robotic thoracic surgery. J Thorac Dis 2021;13:6123-8. [Crossref] [PubMed]
- Andersson SE, Ilonen IK, Pälli OH, et al. Learning curve in robotic-assisted lobectomy for non-small cell lung cancer is not steep after experience in video-assisted lobectomy; single-surgeon experience using cumulative sum analysis. Cancer Treat Res Commun 2021;27:100362. [Crossref] [PubMed]
- Miskovic D, Ahmed J, Bissett-Amess R, et al. European consensus on the standardization of robotic total mesorectal excision for rectal cancer. Colorectal Dis 2019;21:270-6. [Crossref] [PubMed]
- Andolfi C, Umanskiy K. Mastering Robotic Surgery: Where Does the Learning Curve Lead Us? J Laparoendosc Adv Surg Tech A 2017;27:470-4. [Crossref] [PubMed]
- Sasankan P, Chang S, Cerfolio R. Robotic right upper lobectomy: Twelve steps. JTCVS Tech 2021;7:280-2. [Crossref] [PubMed]
- Lefetz O, Baste JM, Hamel JF, et al. Robotic surgery and work-related stress: A systematic review. Appl Ergon 2024;117:104188. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Hubert J, Vouhe P, Poitout D. Rapport 21-13. Formation des chirurgiens/des équipes chirurgicales à la chirurgie robot-assistée. État de la situation actuelle. Propositions d’améliorations. Bull Acad Natle Méd 2022;206:167-78.
- Cerfolio RJ, Cichos KH, Wei B, et al. Robotic lobectomy can be taught while maintaining quality patient outcomes. J Thorac Cardiovasc Surg 2016;152:991-7. [Crossref] [PubMed]
- Siddiqui NY, Galloway ML, Geller EJ, et al. Validity and reliability of the robotic Objective Structured Assessment of Technical Skills. Obstet Gynecol 2014;123:1193-9. [Crossref] [PubMed]
- Goh AC, Goldfarb DW, Sander JC, et al. Global evaluative assessment of robotic skills: validation of a clinical assessment tool to measure robotic surgical skills. J Urol 2012;187:247-52. [Crossref] [PubMed]
- Brook NR, Dell'Oglio P, Barod R, et al. Comprehensive training in robotic surgery. Curr Opin Urol 2019;29:1-9. [Crossref] [PubMed]
- Fernandes E, Elli E, Giulianotti P. The role of the dual console in robotic surgical training. Surgery 2014;155:1-4. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Koupparis A, Villeda-Sandoval C, Weale N, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion: impact on an established enhanced recovery protocol. BJU Int 2015;116:924-31. [Crossref] [PubMed]
- Schiff L, Tsafrir Z, Aoun J, et al. Quality of Communication in Robotic Surgery and Surgical Outcomes. JSLS 2016;20:e2016.00026.
- Geraci TC, Scheinerman J, Chen D, et al. Beyond the learning curve: a review of complex cases in robotic thoracic surgery. J Thorac Dis 2021;13:6129-40. [Crossref] [PubMed]


