
Initial experience of complete portal robotic esophagectomy for esophageal carcinoma in semi-prone position under single-lumen insertion for anaesthesia
Highlight box
Surgical highlights
• Complete portal robotic esophagectomy with 4 arms of Da Vinci surgical system under single-lumen insertion for anaesthesia with artificial pneumothorax in the semi-prone position was a safe and effective technique for surgical treatment of esophagus cancer.
What is conventional and what is novel/modified?
• The conventional surgical technique included left lateral decubitus/prone position combined with single/double-lumen insertion, and 3/4 arms of Da Vinci System with/without a utility incision is widely used.
• Our surgical technique is complete portal robotic esophagectomy with 4 arms of Da Vinci surgical system under single-lumen insertion for anaesthesia with artificial pneumothorax in the semi-prone position.
What is the implication, and what should change now?
• For surgical treatment of patients with esophagus cancer, complete portal robotic esophagectomy with 4 arms of Da Vinci surgical system under single-lumen insertion for anaesthesia with artificial pneumothorax in the semi-prone position could achieve satisfactory short-term outcomes, and it is a safe and effective surgical technique.
Introduction
Esophageal cancer (EC) is the seventh leading cause of cancer-related deaths globally, and esophageal squamous cell carcinoma (ESCC) comprises approximately 90% of all cases (1). Currently, the esophagectomy is still the primary choice to treat EC, which is a complicated surgical procedure that involves excision and reconstruction of the digestive tract (2). Minimally invasive esophagectomy (MIE) has been widely applied in treatment of resectable EC due to fewer complications, faster recovery, and better quality of life than traditional open esophagectomy (3-5). In the early 2000s, robot-assisted MIE (RAMIE) was first applied for the surgical treatment of EC (6,7). Since then, RAMIE has been rapidly developed worldwide, and many studies have demonstrated its safety and feasibility (8-11). Although numerous studies of RAMIE have been reported, there are still no unified surgical approach and technique among surgeons. RAMIE is still a challenging procedure with deep learning curve and further studies are essential to elucidate this technique (12-17).
Herein, we described our initial experience of robotic portal esophagectomy with four arms (RPE-4) with CO2 insufflation, under single-lumen but not double-lumen insertion for anaesthesia in EC patients, using prospective collected data. Using these data, this study aimed to analyse its safety and feasibility, emphasising its advantage in flatting the learning curve of RAMIE to promote the wide spread of this technology for EC patients. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1410/rc).
Preoperative preparations and requirements
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (approval number: B2023-596-01), which is a world-class and domestic leading cancer center in China, Affiliated to Sun Yat-sen University as a Tertiary teaching hospital. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Informed consent for our study was waived because the patients’ information was retrospectively collected and the study did not involve any therapy beyond those routinely performed and administered during standard patient care before, during, and after surgery. Early in June 2018, our team led by Dr. Hao-Xian Yang as attending surgeon began to perform robot-assisted thoracic surgeries, and since then, we have been collecting the data to build a database, as described elsewhere (18-20). The inclusion criteria were as follows: (I) patients pathologically diagnosed with EC; (II) clinical stage T1-3, N0-2, and M0 according to the 8th American Joint Committee on Cancer staging system; and (III) patients who underwent RPE-4. All the included patients received robotic McKeown or Ivor-Lewis esophagectomy using Da Vinci Si/Xi system produced by Intuitive Surgical Inc. from the USA. A comprehensive case report form (CRF) was designed for each included case to collect the data perioperatively (18-20). The cost of time for each step of the operation, and other perioperative factors such as blood loss and conversion, were recorded simultaneously during the operation. Operative complications were recorded and classified according to the Clavien-Dindo classification system (21). The cost of a patient was defined as the total expense from admission to discharge of this patient before reimbursement by Medicare, which was retracted from our Hospital Information System. Then, costs were converted to U.S. dollar ($) with a 2023 intermediate exchange rate of the RMB against the U.S. dollar.
Totally 22 patients were included in this study. Their detailed clinicopathological characteristics are listed in Table 1. The mean age of the entire cohort was 62.4±5.4 years, and 77.3% (17/22) of the patients were male. More than half of the patients had never smoked (12/22, 54.5%). Nine cases (40.9%) had comorbidities, including four with hypertension, one with diabetes and hypertension, one with myocardial infarction, one with hepatitis B, one with depression, and one with chronic obstructive pulmonary disease. Most patients received McKeown esophagectomy (20/22, 90.9%). Squamous cell carcinoma was the predominant histological type (19/22, 86.4%), and most patients were diagnosed with pathological stage II (7/22, 31.8%). Three patients (13.6%) received neoadjuvant therapy, in which two of them achieved pathological complete response.
Table 1
| Characteristics | Values |
|---|---|
| Gender | |
| Male | 17 (77.3) |
| Female | 5 (22.7) |
| Age (years) | |
| Mean ± SD | 62.4±5.4 |
| Median (IQR) | 63.0 (61.0–66.3) |
| Tumor location | |
| Middle third | 14 (63.6) |
| Lower third | 8 (36.4) |
| Comorbidity | |
| No | 13 (59.1) |
| Yes | 9 (40.9) |
| Hypertension | 4 (18.2) |
| Diabetes + hypertension | 1 (4.5) |
| Myocardial infarction | 1 (4.5) |
| Hepatitis B | 1 (4.5) |
| Depression | 1 (4.5) |
| COPD | 1 (4.5) |
| Smoking history | |
| Never | 12 (54.5) |
| Current | 0 |
| Quit | 10 (45.5) |
| Histology | |
| Squamous cell carcinoma | 19 (86.4) |
| Adenocarcinoma | 1 (4.5) |
| Adenosquamous carcinoma | 1 (4.5) |
| Esophageal carcinoma in situ | 1 (4.5) |
| Clinical stage | |
| I | 5 (22.7) |
| II | 4 (18.2) |
| III | 13 (59.1) |
| Pathological stage | |
| 0 | 4 (18.2) |
| I | 5 (22.7) |
| II | 7 (31.8) |
| III | 6 (27.3) |
| Surgical system | |
| Si | 11 (50.0) |
| Xi | 11 (50.0) |
| Surgical type | |
| Ivor-Lewis | 2 (9.1) |
| McKeown | 20 (90.9) |
| Neoadjuvant therapy | |
| No | 19 (86.4) |
| Yes | 3 (13.6) |
Data are presented as n (%) unless otherwise specified. COPD, chronic obstructive pulmonary disease; IQR, interquartile range; RPE-4, robotic portal esophagectomy with 4 arms; SD, standard deviation.
Step-by-step description
General anesthesia method
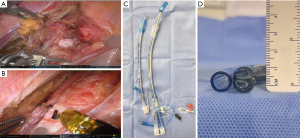
Single-lumen tracheal tube insertion was performed for anaesthesia with the advantages of not only reducing the complexity of the procedure and the potential injury to the tracheal mucosa, but also facilitating the dissection of the lymph nodes (LNs) along the left recurrent laryngeal nerve (RLN), which was shown in Figure 1. As shown in Figure 1A, the space around the trachea of a patient with single-lumen tracheal tube was exposed easily, but according to Figure 1B, the trachea of a patient was obviously swelled by double-lumen tracheal, hampering the dissection of the LNs along the left RLN. In fact, double-lumen tube is significantly larger than the single-lumen tube, and the comparison of these two types of tubes at the panoramic level and at the cross-section level was shown in Figure 1C,1D, respectively.
Patient position and port location strategy
A 4-arm complete robotic portal technique with a 12-mm assistant port was used for all the included patients. The five portal incisions were sufficiently large to match the sizes of the individual trocars. Each adjacent port was made 8–10 cm apart to avoid obstruction between the instruments.
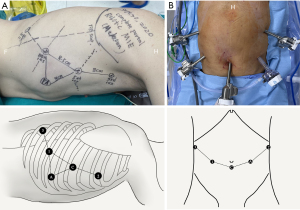
In terms of patient’s position, the patients were placed in a right-side semi-prone position (30°–45°) for the thoracic procedure, as shown in Figure 2A, and the patients were placed in the supine position for the abdominal procedure (Figure 2B). In terms of port location strategy, the port location for the thoracic procedure is shown in Figure 3A. The camera port (12 mm in Si system and 8 mm in Xi system) was placed in the 5th intercostal space (ICS) along the anterior axillary line, and CO2 insufflation was set at 6–8 mmHg. The robot’s left arm (8 mm trocar) was placed in the 8th ICS at the posterior axillary line. The robot’s right arm (8 mm trocar) was placed in the 3rd ICS along the mid-axillary line. The robot’s assistant arm (8 mm trocar) was placed in the 10th ICS along the tip of the scapula line. The port for physician assistant (12 mm trocar) was placed in the 7th ICS at the anterior axillary line. The port location for the abdominal procedure is shown in Figure 3B. The camera port was placed at the bottom of the navel, and CO2 insufflation was performed at 12–14 mmHg. The robot’s left arm was placed 8–10 cm away from the camera port on the left side. The robot’s right arm was placed below the costal arch along the left anterior axillary line. The robot’s assistant arm was placed below the costal arch along the right anterior axillary line. An assistant port was placed between the camera port and robotic right arm.
Thoracic procedure
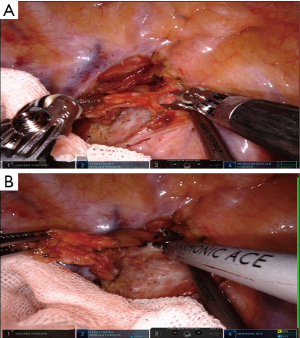
When the robot was docked, robot’s assistant arm was used to ventrally push aside the right-side lung to expose the area of posterior mediastinum. We first divided the pleura at the apex of thoracic cavity using permanent cautery hook combined with the Maryland bipolar forceps or ultrasonic scalpel if necessary to expose and dissect the LNs along the right RLN (Figure 4). Then we divide the upper third of the esophagus along the posterior line of it. For advanced cases, we transect the arch of azygous vein for a better exposure. In the next step, we continue to divide the esophagus along the descending aorta. The proper esophageal artery to the esophagus was transected with robotic ultrasonic scalpel (Figure 5). The thoracic duct was exposed or dissected in advanced cases with suspicious of tumor invasion. During this procedure, the para-esophageal LNs were dissected by en bloc method. After a thorough division, the esophagus was transected above the arch of the azygos vein using a stapler (Figure 6). The distant residual of the esophagus was then lifted for a complete division of the esophagus. By this way, the subcarinal zone could be well exposed and enabled us a convenient LN dissection (Figure 7). The last step of thoracic procedure is dissecting the LNs along the left RLN. The single-lumen tracheal tube insertion during anaesthesia enabled the assistant to push the trachea anteriorly using a suction for a better exposure of the LNs along the left RLN (Figure 8). At last, a chest tube was inserted regularly through the camera port.
Abdominal procedure
When the robot was docked, we first divided the omentum ligament using ultrasonic scalpel until to the short gastric artery (Figure 9). With the help of robot’s assistant arm, the liver could be easily lifted to expose the lesser omentum (Figure 10). After division of the lesser omentum, the left gastric artery and veins could be well exposed and divided using hem-o-lok (Figure 11). After clipping and cutting off the left gastric artery and vein, the lesser curvature was completely divided to the hiatus (Figure 12). During this procedure, LNs alongside the left gastric artery, common hepatic artery, celiac trunk, lesser curvature, and cardia were all completely dissected. Once the whole stomach was fully dissociated, a gastric conduit was created using the stapler from the lesser curve to the fundus (Figure 13). Finally, anastomosis was completed in the neck or thorax. Postoperatively, the patient was transported to the intensive care unit (ICU) with nasogastric and nasoduodenal nutrition tubes.
Here, we presented two surgical videos of two included patients: One underwent robotic McKeown esophagectomy and the other underwent robotic Ivor-Lewis esophagectomy, which was shown in Video 1 and Video 2, respectively.
Postoperative considerations and tasks
The postoperative management of RPE-4 is like that of MIE or open esophagectomy. After RPE-4, patients were transformed from operative room to ICU, and every patient should stay in ICU for at least one night. Chest X-ray was performed on postoperative day (POD) 1 to determine if pulmonary complications occurred. Dual antibiotics were routinely used to control postoperative infections. Gastrointestinal decompression was routinely performed using a nasogastric tube, and the nasogastric tube should be removed before discharge. Total parenteral nutrition (TPN) was performed for 3–5 days after surgery. Then with the increasing amount of enteral nutrition, the amount of TPN could be gradually reduced until no need of TPN, but the nasoduodenal nutrition tube should be kept for at least 2 weeks because patients should keep fasting for at least 2 weeks to avoid anastomotic fistula. Before removing the nasoduodenal nutrition tube, patient should routinely receive an esophageal barium swallow to confirm that no anastomotic fistula occurred. All included patients were followed every 2 weeks using telephone or outpatient follow-up until 90 days after surgeries.
Tips and pearls
In the surgical treatment of EC, complete portal robotic esophagectomy with 4 arms under single-lumen tracheal tube insertion in semi-prone position has advantages in dissection of LNs alongside the left RLN, in reduction of the damage of tracheal mucosa caused by tracheal intubation, in simplifying surgical procedures and flatting the learning curve.
Discussion
With flexible robotic arms and a high-definition three-dimensional view, the robot-assisted surgical system can overcome the inherent defects of traditional endoscopic surgery. Therefore, an increasing number of thoracic surgeons worldwide have adopted RAMIE as a safe and efficient surgical technique (8-11). However, the surgical procedures of esophagectomy are extremely complex, and there is currently no unified standard surgical technique for RAMIE. Therefore, thoracic surgeons from different medical centers should compile their RAMIE surgical techniques to further advance and broaden the application of this method.
In this study, perioperative factors of patients with RPE-4 were summarized in Table 2. All the patients completed RPE-4s successfully, and none of them experienced perioperative death. The median total operative time was 325.0 min [interquartile range (IQR), 296.3–391.3 min], and the median thoracic docking and console time were 7.5 (IQR, 5.0–10.0) and 79.0 (IQR, 65.3–102.3) min, respectively. The median abdominal docking and console time were 7.0 (IQR, 5.0–9.3) and 84.5 (IQR, 67.0–118.8) min, respectively. Besides, the median time of switching patients’ position was 56.0 (IQR, 49.0–60.3) min. The median estimated blood loss during surgery was 100.0 (IQR, 100.0–125.0) mL. The median number of harvested LNs was 28.0 (IQR, 21.3–45.3), and the median length of postoperative stay was 9.0 (IQR, 6.8–12.5) days. The mean number of harvested RLN LNs was 3.6 (median: 2.0), and the achievement rates of the dissection of RLN LNs was 90.9% (20/22). There were no intraoperative complications and conversion to open surgery. Four patients experienced postoperative complications. Patient A had anastomotic fistula on POD 7, then we opened the cervical incision and created a negative pressure suction device. After 20 days, the anastomotic fistula was cured. Patient B had right-side pneumothorax on POD 4, then a closed thoracic drainage was performed, and the pneumothorax disappeared next day. Patient C had left-side pleural effusion on POD 1, then ultrasound-guided thoracentesis was performed and a drainage tube was placed. Finally, the drainage tube was removed on POD 4. Patient D had respiratory failure on POD 1, then the patient was transferred to ICU. After 8 days of symptomatic treatment, patient D was successfully transferred out of ICU. All these four patients were cured and discharged from hospital successfully. No RLN palsy occurred. The median total cost during hospitalization was $20,997.8 (IQR, 18,921.1–24,511.6). According to our results, we demonstrated our experience of RPE-4 with single-lumen anesthesia and CO2 insufflation was a safe and effective surgical technique, which could also flatten the learning curve of RAMIE.
Table 2
| Characteristics | Values |
|---|---|
| Total operative time (min) | |
| Mean ± SD | 341.3±73.9 |
| Median (IQR) | 325.0 (296.3–391.3) |
| Thoracic docking time (min) | |
| Mean ± SD | 7.7±2.7 |
| Median (IQR) | 7.5 (5.0–10.0) |
| Thoracic console time (min) | |
| Mean ± SD | 90.3±34.1 |
| Median (IQR) | 79.0 (65.3–102.3) |
| Time of switching patients’ position (min) | |
| Mean ± SD | 55.6±7.3 |
| Median (IQR) | 56.0 (49.0–60.3) |
| Abdominal docking time (min) | |
| Mean ± SD | 7.3±3.0 |
| Median (IQR) | 7.0 (5.0–9.3) |
| Abdominal console time (min) | |
| Mean ± SD | 96.3±34.9 |
| Median (IQR) | 84.5 (67.0–118.8) |
| Blood loss (mL) | |
| Mean ± SD | 140.7±124.5 |
| Median (IQR) | 100.0 (100.0–125.0) |
| No. of harvested total LNs | |
| Mean ± SD | 33.6±20.0 |
| Median (IQR) | 28.0 (21.3–45.3) |
| No. of harvested right-side RLN LNs | |
| Mean ± SD | 3.6±3.6 |
| Median (IQR) | 2.0 (1.0–6.0) |
| Achievement rates of RLN LNs, n (%) | 20 (90.9) |
| Intraoperative complication | 0 |
| Postoperative complication, n (%) | 4 (18.2) |
| Anastomotic fistula | 1 (4.5) |
| Pneumothorax on right side | 1 (4.5) |
| Pleural effusion on left side | 1 (4.5) |
| Respiratory failure | 1 (4.5) |
| Complication stage | |
| I | 2 (9.1) |
| II | 0 |
| III | 2 (9.1) |
| Conversion, % | 0 |
| Drainage on POD 1 (mL) | |
| Mean ± SD | 367.2±195.4 |
| Median (IQR) | 385.0 (224.8–475.0) |
| Chest tube duration (days) | |
| Mean ± SD | 5.9±2.7 |
| Median (IQR) | 5.5 (3.8-8.0) |
| Length of stay in ICU (days) | |
| Mean ± SD | 2.1±2.0 |
| Median (IQR) | 1.0 (1.0–3.0) |
| Length of postoperative stay (days) | |
| Mean ± SD | 9.9±5.4 |
| Median (IQR) | 9.0 (6.8–12.5) |
| 30-day mortality (%) | 0 |
| 90-day mortality (%) | 0 |
| Cost ($) | |
| Mean ± SD | 22,692.4±7,158.2 |
| Median (IQR) | 20,997.8 (18,921.1–24,511.6) |
ICU, intensive care unit; IQR, interquartile range; LNs, lymph nodes; POD 1, postoperative day 1; RLN, recurrent laryngeal nerve; RPE-4, robotic portal esophagectomy with 4 arms; SD, standard deviation.
Reasonable patient positioning is an important factor for safe and smooth operative progress. The patient position for RAMIE is similar to that of traditional MIE, which mainly includes the left lateral (22-24) and prone positions (13) for the thoracic procedure. In early 2010, Kim et al. proposed RAMIE in the prone position, and their results showed that this technique is technically feasible and safe (13). The patient was placed in a prone position to facilitate mediastinal dissection and minimize lung injury (25). However, Kim et al. also found that the prone position could cause an elevation in central venous pressure and mean pulmonary arterial pressure and a decrease in static lung compliance (13). In addition, prone position is not convenient for conversion to thoracotomy when necessary. Therefore, most thoracic surgeons are more likely to choose the left lateral position as the standard patient position for RAMIE. After comparing the advantages and disadvantages of these two patient positions, we propose the semi-prone position to complete RPE-4. In this position, gravity is used to naturally move the right lung to the ventral side, thereby fully exposing the esophageal region of the posterior mediastinum with the help of assistant arm of Da Vinci System, which was shown in Figure 1A,1B. Additionally, this position does not cause excessive pressure on the heart and lungs and does not damage the patient’s cardiopulmonary function. Even if operative complication occurs, it’s convenient to transfer to open thoracotomy. Based on our study, all 22 patients receiving RAMIE with semi-prone position had satisfactory short-term outcomes. Therefore, we propose that semi-prone position is a good position for RAMIE, but randomized controlled trial (RCT) is essential to compare different positions for operation to draw a definite conclusion.
Regarding the anesthesia intubation method, both double-lumen and single-lumen insertion for anaesthesia have extensive applications in RAMIE, but there is still no definitive conclusion on which of the two techniques is better currently. The advantage of double-lumen tracheal tube is to collapse the lung on the side of surgery to provide a capacious surgical field, and to effectively prevent the secretions and blood of the affected lung from contaminating the healthy lung. However, the double-lumen tracheal tube is larger in diameter than the single-lumen tracheal tube, which could cause greater damage to the patient’s tracheal mucosa, leading to cough and lung infection after surgery. Besides, double-lumen tracheal tube insertion is a more challenging technique, which is time-consuming and energy-draining. An additional benefit of single-lumen tracheal tube insertion is cost savings for patients. In our hospital, one double-lumen tracheal tube costs $300 more than one single-lumen tracheal tube. In mainland China, the total cost of esophagectomy is only about $1,000, and mainland China accounts for more than half of the world’s EC patients. Therefore, saving $300 per patient is not a small amount. More importantly, single-lumen tracheal tube insertion however, is easy to complete for anaesthesiologists and is beneficial for exposing the tracheoesophageal groove region and protecting intraoperative lung function (25,26). With the assistance of low tidal volume and artificial pneumothorax, the surgical field of view was not significantly restricted (as shown in our videos), and the surgical procedure remained smooth. Based on our results, the mean number of harvested RLN LNs was 3.6, and the achievement rate of the dissection of RLN LNs was 90.9%, indicating the feasibility and efficiency of our surgical technique. Of course, RCT is essential to compare single-lumen insertion and double-lumen insertion for anesthesia in RAMIE.
In terms of short-term outcomes, it should be noted that the number of resected LNs in our study (mean: 33.6) was much more than those of ROBOT trial (mean: 27) (27) and RAMIE trial (mean: 23) (8). Regarding the perioperative complications, the overall complication rate was lower in our study (18.2%) compared with ROBOT trial (59%) (27) and RAMIE trial (48.6%) (8). More importantly, there was only one patient having anastomotic fistula and no one had hoarseness after surgery. Although the patients included in our study were the first 22 cases treated by RPE-4, but the number of examined LNs in our study was more than those of ROBOT trial and RAMIE trial with acceptable operative time. More harvested LNs in this study but no patient having hoarseness after surgery suggests that our technique could not only guarantee the completeness of surgery, but might also reduce the surgical complications, especially for the protection of RLN. It is worth noting that although there were only 22 cases included in this study, during the study period, our surgical team also performed robotic lobectomies (18), robotic segmentectomies (20), and robotic mediastinal tumor resection (28). Additionally, in clinical practice, whether three-port or four-port RAMIE is adopted depends on several factors, including the surgeon’s technical expertise, as well as the economic conditions of the region. For instance, in mainland China, RAMIE is not yet covered by medical insurance. To economize costs, many surgeons from mainland China choose to use three robotic arms instead of four, supplemented by an additional incision of approximately 3–4 cm to facilitate the smooth progression of the surgery (15,29).
There are some limitations in this study. First, this was a single-arm study that only included 22 patients with RPE-4, and whether this technique was superior to conventional MIEs or other types of RAMIEs needs further investigation. Second, all included RPE-4s were performed by an experienced team in MIE in large volume cancer center, and the results might differ from those of other surgical teams. Third, this study only explored the short-term outcomes of RPE-4, and the long-term outcomes of this technique need to be further investigated.
Conclusions
In conclusion, this study demonstrated that RPE-4 under single-lumen tracheal tube insertion, CO2 insufflation, and semi-prone position for thoracic procedure is a safe and effective technique for MIE in EC patients. It has advantages in LN dissection, reducing complications, and flattening the learning curve with satisfied short-term outcomes. Further prospective comparative trials are warranted to testify our results.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1410/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1410/prf
Funding: This work was supported in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (approval number: B2023-596-01). All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Informed consent for our study was waived because the patients’ information was retrospectively collected and the study did not involve any therapy beyond those routinely performed and administered during standard patient care before, during, and after surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Horgan S, Berger RA, Elli EF, et al. Robotic-assisted minimally invasive transhiatal esophagectomy. Am Surg 2003;69:624-6.
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: the RAMIE Trial. Ann Surg 2022;275:646-53. [Crossref] [PubMed]
- Mederos MA, de Virgilio MJ, Shenoy R, et al. Comparison of Clinical Outcomes of Robot-Assisted, Video-Assisted, and Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2021;4:e2129228. [Crossref] [PubMed]
- Tagkalos E, Goense L, Hoppe-Lotichius M, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity-matched analysis. Dis Esophagus 2020;33:doz060. [Crossref] [PubMed]
- Tsunoda S, Obama K, Hisamori S, et al. Lower Incidence of Postoperative Pulmonary Complications Following Robot-Assisted Minimally Invasive Esophagectomy for Esophageal Cancer: Propensity Score-Matched Comparison to Conventional Minimally Invasive Esophagectomy. Ann Surg Oncol 2021;28:639-47. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Kim DJ, Hyung WJ, Lee CY, et al. Thoracoscopic esophagectomy for esophageal cancer: feasibility and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg 2010;139:53-59.e1. [Crossref] [PubMed]
- Kingma BF, Grimminger PP, van der Sluis PC, et al. Worldwide Techniques and Outcomes in Robot-assisted Minimally Invasive Esophagectomy (RAMIE): Results From the Multicenter International Registry. Ann Surg 2022;276:e386-92. [Crossref] [PubMed]
- Zhang X, Su Y, Yang Y, et al. Robot assisted esophagectomy for esophageal squamous cell carcinoma. J Thorac Dis 2018;10:3767-75. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Finley DJ, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg 2013;43:e107-15. [Crossref] [PubMed]
- Yang MZ, Lai RC, Abbas AE, et al. Learning curve of robotic portal lobectomy for pulmonary neoplasms: A prospective observational study. Thorac Cancer 2021;12:1431-40. [Crossref] [PubMed]
- Yang MZ, Tan ZH, Li JB, et al. Comparison of Short-Term Outcomes Between Robot-Assisted and Video-Assisted Segmentectomy for Small Pulmonary Nodules: A Propensity Score-Matching Study. Ann Surg Oncol 2023;30:2757-64. [Crossref] [PubMed]
- Yang MZ, Tan ZH, Abbas AE, et al. Defining the learning curve of robotic portal segmentectomy in small pulmonary lesions: a prospective observational study. J Robot Surg 2023;17:1477-84. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Abbott A, Shridhar R, Hoffe S, et al. Robotic assisted Ivor Lewis esophagectomy in the elderly patient. J Gastrointest Oncol 2015;6:31-8. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. vii. [Crossref] [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus vs. prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Gan W, Yang MZ, Tan ZH, et al. Robotic portal resection for mediastinal tumours: a prospective observational study. J Cardiothorac Surg 2024;19:155. [Crossref] [PubMed]
- Deng HY, Huang WX, Li G, et al. Comparison of short-term outcomes between robot-assisted minimally invasive esophagectomy and video-assisted minimally invasive esophagectomy in treating middle thoracic esophageal cancer. Dis Esophagus 2018;31: [Crossref] [PubMed]












