
Neoadjuvant arterial infusion chemotherapy combined with immunotherapy in treating locally advanced lower esophageal and esophagogastric junction cancer
Highlight box
Key findings
• A small case series indicates that neoadjuvant arterial infusion chemotherapy combined with immunotherapy (neo-AICIT) demonstrates favorable safety and efficacy in treating locally advanced lower esophageal/esophagogastric junction cancers.
What is known and what is new?
• The treatment of locally advanced lower esophageal cancer/esophagogastric junction cancers has faced certain challenges and limitations.
• This manuscript adds a novel neoadjuvant therapeutic approach, showing that neo-AICIT results in major pathological remission and high objective response rate, with only grade 1 treatment-related adverse events and specific postoperative complications.
What is the implication, and what should change now?
• Neo-AICIT holds potential as a promising neoadjuvant treatment option. Future large-scale studies are necessary to validate and optimize this treatment modality for wider application.
Introduction
The incidence of esophageal cancer is high in China. Radical surgical treatment is one of the most important therapies for esophageal cancer. However, 40.7% of patients diagnosed with esophageal cancer were already in advanced or locally advanced stage upon the diagnosis (1,2), and lost the opportunity for surgical treatment. For the locally advanced esophageal cancer and esophagogastric junction cancers, many clinical studies have shown that neoadjuvant chemoradiotherapy plus surgery is superior to surgery alone (3-5), both the guidance of the National Comprehensive Cancer Network (NCCN) (6) and the guidance of the Chinese Society of Clinical Oncology (CSCO) (7) also recommend performing neoadjuvant chemoradiotherapy and then surgery evaluation. With the arrival of the immune era, several studies on the treatment of locally advanced esophageal cancer with neoadjuvant chemotherapy combined with immunotherapy or chemoradiotherapy combined with immunotherapy also showed good prospect (8-14). In the TD-NICE study (15), tislelizumab, albumin paclitaxel, and carboplatin were used as a neoadjuvant therapy. The pathological complete response (pCR) rate reached 50%. In the PALACE-1 study (16), pembrolizumab, carboplatin, paclitaxel, and radiotherapy were used as a neoadjuvant therapy. The pCR rate reached 55.6%.
However, neoadjuvant chemoradiotherapy or chemotherapy combined with immunotherapy is not applicable to all patients with locally advanced esophageal cancer. For instance, many patients with advanced esophageal cancer are elderly patients and have poor tolerance to concurrent chemoradiotherapy or chemotherapy. During the treatment, these patients discontinue the treatment or reduce the dose of anticancer drug due to their intolerance to the chemotherapy-caused toxicities and adverse events. In addition, for some patients with lower esophageal cancer or esophagogastric junction cancer complicated with abdominal lymph node metastasis, if radiotherapy is performed simultaneously, the radiation will pass through the stomach, which may affect the healing of esophagogastric anastomosis in a later operation. For the above-mentioned problem, the neoadjuvant arterial infusion chemotherapy combined with immunotherapy (neo-AICIT) has been proposed to reduce the side effects of neoadjuvant therapy.
The research on interventional treatment of liver tumors has long confirmed that arterial infusion chemotherapy can increase the drug concentration in the tumor area 5–20 times higher than that in normal liver tissue, and 100–400 times higher than that throughout the body, which not only effectively controls the tumor, but also greatly reduces the systemic toxicities and adverse events (17). Currently, arterial infusion chemotherapy combined with immunotherapy has been applied in the treatment of liver cancer, and reports have shown its good efficacy and safety (18). However, there are still few reports on arterial infusion chemotherapy for esophageal cancer. The study carried out by Liu et al. (19) reported a relatively large number of cases, with 46 patients studied. They were treated with cisplatin or paraplatin and 5-fluorouracil infusion chemotherapy. After 2–4 cycles of perfusion chemotherapy, patients’ objective response rate (ORR) reached 89.3%. In another report by Yin et al. (20), 75 cases were studied. Pharmorubicin, oxaliplatin, and raltitrexed were used as infusion chemotherapy. After three cycles of treatment, the ORR reached 94.7%. This is consistent with our observation in previous clinical work that arterial infusion chemotherapy for esophageal cancer not only has excellent effects but also has minimal side effects and good patient tolerance.
Since the blood supply for the lower esophageal and cardia is mainly from the left gastric artery and inferior phrenic artery, arterial perfusion is relatively easy to operate. Therefore, patients with lower esophageal cancer or esophagogastric junction cancers were selected as study subjects. In addition, the left gastric artery comes from the celiac trunk artery and the blood flow from the celiac trunk artery also supplies some upper abdominal lymph nodes. Injecting chemotherapeutic drugs into left gastric artery and trunk abdominal artery can cover lymph nodes in the lower esophagus, cardia, and epigastrium with high concentrations of chemo drugs.
Now, there is no report on the neo-AICIT for locally advanced lower esophageal cancer and esophagogastric junction cancers. In Xiamen Humanity Hospital of Fujian Medical University, the neo-AICIT has been performed on patients with locally advanced lower esophageal cancer and esophagogastric junction cancers since June 2021. The tumor regression was followed by thoracoscopic radical resection of esophageal cancer, after which 1 year of immunomonotherapy was maintained. The retrospective summary of the patients who received this therapy is as follows. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1908/rc).
Methods
Ethics statement
This study was approved by the Medical Ethics Committee of Xiamen Humanity Hospital of Fujian Medical University (No. HAXM-MEC-20230804-029-01). This study was conducted in accordance with the provisions of the Declaration of Helsinki (as revised in 2013). Considering its retrospective design, the requirement of informed consent from each patient was waived by the ethics committee. The trial was registered in the Chinese Clinical Trial Registry (ChiCTR) with the identifier ChiCTR2300074690.
Cases collected
The data of patients who were hospitalized in Xiamen Humanity Hospital of Fujian Medical University and were treated with neo-AICIT for locally advanced lower esophageal cancer or esophagogastric junction cancers from October 2021 to May 2023 were collected. Inclusion criteria: (I) patients who were diagnosed with cT3N0–2M0 III lower esophageal cancer or esophagogastric junction cancer by gastroscopic biopsy, positron emission tomography (PET)-computed tomography (CT) and other auxiliary examinations; (II) patients who received neo-AICIT; (III) patients who underwent radical surgery for esophageal cancer; and (IV) patients who maintained 1 year of immunomonotherapy after the surgery. Exclusion criteria: (I) patients who were found with a variation of supplying arteries in the lower esophagus or cardiac area during interventional operation, which may greatly influence the efficacy; (II) patients who abandoned the treatment after only ≤2 courses of neoadjuvant therapy, with the efficacy unevaluated; (III) patients who rejected efficacy evaluation for various reasons or were lost to follow-up after neoadjuvant therapy.
Regimen of neoadjuvant therapy
Tislelizumab [Beigene (Shanghai) Biotechnology Co., Ltd., Shanghai, China; 100 mg/piece] 200 mg by intravenous infusion on day 1; docetaxel (Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Qinhuangdao, China; 20 mg/piece) 75 mg/m2 + cisplatin (Jiangsu Hanosen Pharmaceutical Group Co., Ltd., Lianyungang, China; 30 mg/piece) 75 mg/m2 by injection into left gastric artery and trunk abdominal artery for chemotherapy on day 1. This regimen was repeated every 21 days. The efficacy was evaluated after two cycles of treatment. There might be the following three circumstances after evaluation: (I) the neoadjuvant regimen was well tolerated by patients and the reexamination indicated that the regimen was effective. In such case, four cycles of neoadjuvant therapy were completed and then the operation was evaluated. (II) Patients could not tolerate the side effects of chemotherapy and immunotherapy, but the reexamination indicated that the treatment was effective and evaluated that the R0 resection of the tumor was possible. In such cases, the neoadjuvant therapy was stopped; and patients underwent the operation. (III) No matter whether patients tolerated the neoadjuvant therapy or not, the reexamination indicated tumor progression and ineffective treatment. The neoadjuvant therapy plan was discontinued. The treatment plan was changed upon discussion by the oncology multidisciplinary team (see Figure 1).

Intra-arterial infusion chemotherapy
After local anesthesia, a right femoral artery puncture was performed. A 5-F introducer sheath was implanted and the 5-F retrograde left gastric (5F-RLG) catheter was inserted, with the catheter opening placed in the celiac axis. The microcatheter was super-selectively inserted into the left gastric artery and 1/2 dose of cisplatin solution (calculated by the patient’s body surface area and dissolved in 150 mL of normal saline) was slowly injected via the microcatheter. Afterward, the microcatheter was washed with 10 mL of normal saline. Next, 1/2 dose of docetaxel solution (calculated by the patient’s body surface area and dissolved in 150 mL of normal saline) was slowly injected; and then the microcatheter was washed with 10 mL of normal saline. The microcatheter was removed. The opening of the 5F-RLG catheter was placed in the celiac axis. The remaining 1/2 dose of cisplatin solution and docetaxel solution were injected via the catheter. The catheter was removed. The operation ended. The femoral artery puncture point was pressed for 24 h (see Figures 2,3).

Radical resection of esophageal cancer under combined thoracoscope and laparoscope
For patients with tumor regression after neoadjuvant therapy and possible R0 resection as evaluated by CT images. The thoracoscopic radical resection of esophageal carcinoma (McKeown operation) was performed 4–6 weeks after the last intravenous infusion of tislelizumab. Thoracic esophagectomy and mediastinal lymph node dissection were first completed via the four 0.5–1 cm small incisions made in the right chest wall in the left lateral position during the operation [lymph node regions according to the 8th edition of American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) Tumor-Node- Metastasis (TNM) Staging for Cancer of the Esophagus and Esophagogastric Junction, lymph nodes in groups 2, 4, 7, 8, 9 and bilateral recurrent laryngeal nerve lymph nodes were dissected]. Next, the stomach and abdominal esophagus were dissociated and the celiac lymph nodes (groups 1 to 10 lymph nodes) were dissected via the five 0.5–1 cm small incisions made in the abdomen in the supine position. The cervical esophagus was then dissociated and cut off through a 5 cm incision in the left neck. A 6 cm incision was made under the abdominal xiphoid process and the esophagus and stomach were pulled out via the incision. The stomach was shaped into a tube along the greater curvature and pulled to the neck and esophageal stump anastomosis.
Postoperative treatment and management
Postoperative complications (if any) were managed by symptomatic treatment according to the treatment norms. Anastomotic fistula and delayed gastric emptying were excluded. Food intake was gradually resumed 7–10 days after the operation. One month after the operation, when immunotherapy contraindications were excluded (judged according to drug instructions), the immunotherapy was maintained: tislelizumab, 200 mg by intravenous drip. This regimen was repeated every 21 days and the treatment was maintained for 1 year. Regular reexaminations of Chest and abdomen enhanced CT, cervical lymph node color ultrasound, blood squamous cell carcinoma antigen, and carcinoembryonic antigen (CEA) were performed. Reexaminations were performed every 3 months within 2 years after the operation. If no tumor recurrence and metastasis are found, reexaminations will be performed every 6 months in the 3rd to 5th years after the operation and then annually. In case of tumor recurrence and metastasis found during follow-up, the subsequent regimen will be determined upon discussion by the oncology multidisciplinary team.
Observation indicators
- Basic information of patients: gender, age, Eastern Cooperative Oncology Group (ECOG) score, pathological type of tumor, tumor site (lower esophagus or esophagogastric junction), clinical staging of tumor (according to NCCN guidance), programmed death ligand 1 (PD-L1) expression, and underlying diseases. The expression of PD-L1 on tumor cells was expressed as tumor proportion score (TPS).
- Treatment-related indicators: complications of the interventional operation, treatment-related adverse events (TRAEs), the effect of neoadjuvant therapy, operative complications, progression-free survival (PFS), and follow-up time.
Efficacy of neoadjuvant therapy: all patients were evaluated by modified Response Evaluation Criteria in Solid Tumors (mRECIST) (21) and tumor regression grading (TRG) (22).
Per the mRECIST, tumor response is assessed as follows: complete response (CR) refers to the disappearance of any intratumoral arterial enhancement in all target lesions; partial response (PR) entails at least a 30% decrease in the sum of the diameters of viable (arterial phase enhancement) target lesions; progressive disease (PD) entails an increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions; stable disease (SD) is any case that does not qualify for PR or PD.
The TRG was divided into five grades. TRG 1: indicates no residual tumor; TRG 2: indicates scattered cancer cells in fibrous tissue (residual tumor <10%); TRG 3: indicates more fibrous tissue than residual cancer cells (residual tumor 10–50%); TRG 4: indicates less fibrous tissue than residual cancer cells (residual tumor >50%); TRG 5: indicates no regression at all. pCR and major pathological remission (MPR) are considered equivalent to TRG 1 and TRG 1–2, respectively.
Statistical analysis
Age and follow-up time were presented with the median. The incidence TRAEs, the efficacy of neoadjuvant therapy and TRG were presented with percentages. No statistical software was used.
Results
Basic information
A total of 14 patients received neoadjuvant therapy. Among them, two patients achieved a CR in the imaging evaluation after completing four cycles of treatment. However, due to the fear of surgery, they chose to receive radical radiotherapy. Twelve patients completed the four cycles of neoadjuvant therapy and then underwent thoracoscopic and laparoscopic radical resection of esophageal cancer, after which the immune maintenance therapy was completed as scheduled.
All of the 12 patients were male aged 51–72 years with a median age of 61.5 years. The performance status of ECOG score of them was 0 to 1. The histological type of all patients was diagnosed as squamous cell carcinoma; eight of them had lower esophageal cancer and four had esophagogastric junction cancer. The clinical staging in all patients was cT3N0–2M0G1–3. Four patients were complicated with hypertension and three patients with diabetes.
Complications of neo-AICIT
The interventional operation was successfully performed on all patients. No vascular variation was found. Except for low fever in one patient, which was improved after the treatment with a non-steroidal drug (etoricoxib, 60 mg, oral administration, once/day for 3 days), no other complications associated with endovascular intervention occurred.
During the neoadjuvant therapy, loss of appetite without nausea or vomiting occurred in three patients and was improved without any treatment around 5 days later. Grade I myelosuppression manifested as decreased white blood cell count occurred in five patients and was restored 1 week later. Loss of appetite and myelosuppression simultaneously occurred in one patient. The incidence of grade 1 TRAEs was 58.3% (7/12).
Surgical complications
After the operation, two patients had hoarseness due to recurrent laryngeal nerve injury (Clavien-Dindo classification 1). The postoperative laryngoscopy confirmed that it was caused by right vocal cord paralysis. The pronunciation was found improved during the follow-up, but it was not completely recovered. Two patients were complicated with postoperative pulmonary infection (Clavien-Dindo classification 2), which was improved after anti-infective therapy. The incidence of operative complications was 33.3% (4/12).
Effect of neoadjuvant therapy
The postoperative pathology showed that 11 (91.7%, 11/12) patients reached MPR, TRG 1–2. Among them 7 (58.3%, 7/12) patients reached pCR, TRG 1. One (8.33%, 1/12) patient reached TRG 3. The ORR was 100%.
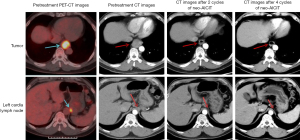
All patients were followed up for 13–24 months, with a median follow-up time of 19.5 months. During the follow-up, two patients had mediastinal lymph node metastasis at 18 and 20 months after operation, respectively. Both patients were confirmed by ultrasound bronchoscopic biopsy as tumor metastasis and received radiotherapy. No tumor recurrence or metastasis was found in the remaining patients (see Tables 1,2 and Figures 4,5).
Table 1
| No. | Age (years)/gender | ECOG status | Pathological types | Tumor site | Solid tumor stage | TPS (%) |
Basic disease | Interventional procedures complications | TRAEs | mRECIST | TRG (grade) | Operative complication | PFS (mon) |
Postoperative follow-up time (mon) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70/M | 1 | Squamous | Lower esophagus | cT3N1M0G2–3 | 95 | Hypertension | No | Decreased appetite | CR | 1 pCR | No | NR | 30 |
| 2 | 60/M | 0 | Squamous | Lower esophagus | cT3N2M0G1 | 35 | No | Low-grade fever | Myelosuppression (grade I) | PR | 2 MPR | No | 18 | 28 |
| 3 | 58/M | 0 | Squamous | EGJ | cT3N0M0G2 | 2 | No | No | No | CR | 1 pCR | Pulmonary infection | NR | 26 |
| 4 | 65/M | 0 | Squamous | Lower esophagus | cT3N1M0G1 | 75 | Hypertension | No | No | CR | 1 pCR | No | NR | 25 |
| 5 | 51/M | 0 | Squamous | EGJ | cT3N1M0G2 | 50 | No | No | Myelosuppression (grade I), decreased appetite | CR | 1 pCR | Recurrent laryngeal nerve injury | 20 | 24 |
| 6 | 68/M | 1 | Squamous | Lower esophagus | cT3N1M0G1 | 0 | No | No | No | PR | 2 MPR | Recurrent laryngeal nerve injury | NR | 21 |
| 7 | 72/M | 1 | Squamous | Lower esophagus | cT3N2M0G2 | 15 | Hypertension, diabetes | No | Myelosuppression (grade I) | CR | 1 pCR | No | NR | 18 |
| 8 | 59/M | 0 | Squamous | Lower esophagus | cT3N1M0G1 | 80 | Diabetes | No | No | CR | 1 pCR | No | NR | 15 |
| 9 | 63/M | 0 | Squamous | Lower esophagus | cT3N0M0G1 | 0 | Hypertension | No | Decreased appetite | PR | 2 MPR | No | NR | 15 |
| 10 | 55/M | 0 | Squamous | Lower esophagus | cT3N1M0G1 | 55 | No | No | Myelosuppression (grade I) | CR | 1 pCR | No | NR | 13 |
| 11 | 64/M | 0 | Squamous | EGJ | cT3N1M0G2 | 30 | Diabetes | No | No | PR | 2 MPR | No | NR | 12 |
| 12 | 57/M | 0 | Squamous | EGJ | cT3N1M0G2 | 10 | No | No | Myelosuppression (grade I) | PR | 3 | Pulmonary infection | NR | 12 |
Solid tumor stage: according to the NCCN guidelines. Solid tumor stage: according to the NCCN guidelines. CR, complete response; ECOG, Eastern Cooperative Oncology Group; EGJ, esophagogastric junction; M, male; mon, month; MPR, major pathological remission; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NCCN, National Comprehensive Cancer Network; NR, not reach; PD-L1, programmed death ligand 1; PFS, progression-free survival; pCR, pathological complete response; PR, partial response; TPS, tumor proportion score (PD-L1 expression on tumor cells); TRAEs, treatment-related adverse events; TRG, tumor regression grade.
Table 2
| Characteristics | Data (n=12) |
|---|---|
| Age (years) | 61.5 [51–72] |
| Sex | |
| Male | 12 (100.0) |
| Female | 0 |
| ECOG status | |
| 0 | 9 (75.0) |
| 1 | 3 (25.0) |
| Pathological types | |
| Squamous | 12 (100.0) |
| Adenocarcinoma | 0 |
| Tumor site | |
| Lower esophagus | 8 (66.7) |
| EGJ | 4 (33.3) |
| TPS (%) | |
| <1 | 2 (16.7) |
| 1–49 | 5 (41.7) |
| ≥50 | 5 (41.7) |
| Basic disease | |
| Hypertension | 4 (33.3) |
| Diabetes | 3 (25.0) |
| No | 5 (41.7) |
| Interventional procedures complications | |
| Low-grade fever | 1 (8.3) |
| No | 11 (91.7) |
| TRAEs† | |
| Decreased appetite | 3 (25.0) |
| Myelosuppression (grade I) | 5 (41.7) |
| No | 5 (41.7) |
| mRECIST | |
| CR | 7 (58.3) |
| PR | 5 (41.7) |
| Other | 0 |
| TRG (grade) | |
| 1 | 7 (58.3) |
| 2 | 4 (33.3) |
| 3 | 1 (8.3) |
| Other | 0 |
| Pathological response | |
| pCR | 7 (58.3) |
| MPR | 11 (91.7) |
| No-MPR | 1 (8.3) |
| Operative complication | |
| Pulmonary infection | 2 (16.7) |
| Recurrent laryngeal nerve injury | 2 (16.7) |
| No | 8 (66.7) |
| Postoperative follow-up time (months) | 19.5 [13–24] |
Data are presented as median [range] or n (%). †, some patients had two types of TRAEs simultaneously. Solid tumor stage: according to the NCCN guidelines. CR, complete response; ECOG, Eastern Cooperative Oncology Group; EGJ, esophagogastric junction; MPR, major pathological remission; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NCCN, National Comprehensive Cancer Network; PD-L1, programmed death ligand 1; pCR, pathological complete response; PR, partial response; TPS, tumor proportion score (PD-L1 expression on tumor cells); TRAEs, treatment-related adverse events; TRG, tumor regression grade.


Discussion
The neo-AICIT for locally advanced esophageal cancer is one of the popular subjects in esophageal surgery. Relevant studies have shown that neo-AICIT may be superior to traditional neoadjuvant concurrent chemoradiotherapy, while the neoadjuvant concurrent chemoradiotherapy combined with immunotherapy may obtain a better effect that the neo-AICIT (5-14). Although almost all studies have shown that the addition of immunotherapy on the basis of neoadjuvant chemotherapy or chemoradiotherapy does not significantly increase the side effects of drugs, the incidence of TRAEs and serious adverse events (SAEs) reached 71.95–91.6% and 16.95–19.4%, respectively (23,24). TRAEs still are important factors affecting the implementation and effect of neoadjuvant therapy. Discontinuing treatment or reducing drug or radiation dose is common in clinical practices due to patients’ intolerance to the side effects of drugs.
Arterial infusion chemotherapy for esophageal cancer is rarely applied in clinical practices, and there are few relevant reports. Compared with traditional intravenous chemotherapy, its advantage lies in the infusion chemotherapy through the blood supply artery of the esophageal tumor. On the one hand, the blood drug concentration in the tumor area can be greatly improved to kill tumor cells more effectively. On the other hand, the blood drug concentration in systemic circulation can be reduced to relieve the side effects of chemotherapy drugs. It is an ideal chemotherapy method for locally advanced esophageal cancer without distant metastasis.
Whether the neo-AICIT for locally advanced esophageal cancer can obtain the same or even better effect as or than conventional intravenous chemotherapy combined with immunotherapy while reducing TRAEs. There is no relevant published report. Xiamen Humanity Hospital of Fujian Medical University began to perform neoadjuvant arterial infusion chemotherapy for locally advanced lower esophageal cancer and esophagogastric junction cancers in 2018 (whether the therapy is combined with concurrent radiotherapy is determined by patients’ physical conditions). Arterial infusion chemotherapy is preferred especially for patients with advanced age, Karnofsky score <90 points, or ECOG score ≥1 point. Xiamen Humanity Hospital of Fujian Medical University began to try the neo-AICIT for locally advanced lower esophageal cancer and esophagogastric junction cancers in 2021. At present, the main reasons for the limitation of this therapy to lower esophageal cancer and esophagogastric junction cancer are the relatively fixed arterial blood supply and thick blood vessels at the lower esophagus and esophagogastric junction, which facilitate interventional operation. Even such complications as vascular occlusion caused by vascular injury during the interventional operation will not influence subsequent operations. However, the arterial blood supply in the middle and upper esophagus has more variations and is thinner, making the interventional operation difficult. Moreover, vascular injury may influence the blood supply of esophagogastric anastomosis during subsequent operation and cause an anastomotic fistula. Since multiple studies (8-14) have shown that regardless of the expression of PD-L1, the addition of PD-L1 inhibitor can improve the efficacy, all patients in this study were treated with PD-L1 inhibitor.
In this study, all 12 patients completed the complete treatment and follow-up plans. The interventional operation was successfully performed on all patients. The neoadjuvant therapy was well tolerated by patients. 58.3% (7/12) of patients had a short-term loss of appetite and grade I myelosuppression, which were spontaneously relieved. There were no TRAEs above grade 2. Incidence of TRAEs is lower than most reports of neoadjuvant chemotherapy combined with immunotherapy for esophageal cancer (23,24). All patients were operated as scheduled. R0 resection was performed. The operation was not influenced by such TRAEs as side effects of drugs. The operation was not made much more difficult. Postoperative complications included pulmonary infection 16.7% (2/12) and recurrent laryngeal nerve injury 16.7% (2/12), relative to reports of neoadjuvant chemotherapy combined with immunotherapy for esophageal cancer (23,24), are not increased. Theoretically, arterial infusion chemotherapy may cause gastrointestinal toxicity, blood flow disorders, and scar formation, thereby leading to increased incidences of decreased appetite, gastric emptying disorders, and anastomotic fistula after surgery (23,24). However, in this study, none of the patients presented these conditions. This might be because we chose to perform the chemotherapy infusion at the celiac trunk artery, where the drugs reached the lesser curvature of the stomach, the cardia, and the lower part of the esophagus through the left gastric artery from the celiac trunk artery. Most of these areas were located in the regions to be resected during the surgery, so the related postoperative complications were not obvious. This indicates that the neo-AICIT is highly safe. The operation pathology showed that 91.66% (11/12) of patients reached MPR, while 58.3% (7/12) of patients reached pCR, the ORR was 100%, which is higher than most reports of neoadjuvant chemotherapy combined with immunotherapy for esophageal cancer (25-36). During the 12–30 months of postoperative follow-up, tumor progression was found in only two patients. Recurrence or metastasis has still not been detected in the remaining patients.
According to the 12 cases, the effect of the neo-AICIT is encouraging. It preliminarily confirms our hypothesis and expectation of neoadjuvant arterial infusion chemotherapy combined with immunotherapy for locally advanced esophageal cancer. Small sample observational data show that compared with conventional neoadjuvant chemotherapy combined with immunotherapy (25-36), neo-AICIT has fewer TRAEs, better tumor shrinkage effect, and does not increase the risk of surgery.
It should be stressed that although the data from this study is amazing, they are the results of just a small sample and retrospective experience summary. The follow-up last for only a short time. Whether the effect of the neoadjuvant therapy can be converted to overall survival (OS) is still unclear. Whether locally advanced middle and upper esophageal cancer can benefit from the neo-AICIT is still unknown. Due to the relatively low number of esophageal cancer patients treated at Xiamen Humanity Hospital of Fujian Medical University, it is challenging for us to conduct large-scale prospective studies. Therefore, we have specifically reported our findings with the hope of arousing interest among our colleagues working in well-equipped major centers, and conducting multi-center large-sample prospective studies to more comprehensively validate the safety and advantages of neo-AICIT.
Conclusions
Observations of small case series suggest that neo-AICIT has good safety and efficacy in the treatment of locally advanced lower esophageal/esophagogastric cancer, and may be a promising neoadjuvant treatment option.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1908/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1908/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1908/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1908/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Xiamen Humanity Hospital of Fujian Medical University (No. HAXM-MEC-20230804-029-01). This study was conducted in accordance with the provisions of the Declaration of Helsinki (as revised in 2013). Considering its retrospective design, the requirement of informed consent from each patient was waived by the ethics committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Early Diagnosis and Treatment Group of the Chinese Medical Association Oncology Branch. Chinese expert consensus on early diagnosis and treatment of esophageal cancer. Zhonghua Zhong Liu Za Zhi 2022;44:1066-75. [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Gao T, Yang Y, Zhang Z, et al. A Surrogate Endpoint for Overall Survival in Locally Advanced and Resectable Esophageal Squamous Cell Carcinoma: A Reanalysis of Data From the NEOCRTEC5010 Trial. Int J Radiat Oncol Biol Phys 2023;117:809-20. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers. Version 2. 2025. Available online: https://www.nccn.org/guidelines/guidelinesdetail?category=1&id=1433
- Chinese Society of Clinical Oncology Guidelines Working Committee. Esophageal Cancer Diagnosis and Treatment Guidelines. Version 2023. Available online: http://www.csco.org.cn/
- Dennehy C, Khan AF, Zaidi AH, et al. The Evolving Landscape of Neoadjuvant Immunotherapy in Gastroesophageal Cancer. Cancers (Basel) 2024;16:286. [Crossref] [PubMed]
- Zeng H, Zhang F, Sun Y, et al. Treatment options for neoadjuvant strategies of esophageal squamous cell carcinoma Mol Clin Oncol 2024;20:4. (Review). [Crossref] [PubMed]
- Zhu M, Yoon HH. Neoadjuvant Immunotherapy in Gastroesophageal Cancer: A Promising Early Signal? J Clin Oncol 2024;42:373-7. [Crossref] [PubMed]
- Wang H, Song C, Zhao X, et al. Evaluation of neoadjuvant immunotherapy and traditional neoadjuvant therapy for resectable esophageal cancer: a systematic review and single-arm and network meta-analysis. Front Immunol 2023;14:1170569. [Crossref] [PubMed]
- Qin H, Liu F, Zhang Y, et al. Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis. Front Immunol 2023;14:1108213. [Crossref] [PubMed]
- He W, Wang C, Li C, et al. The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis. Front Immunol 2023;14:1118902. [Crossref] [PubMed]
- Liu Y, Bao Y, Yang X, et al. Efficacy and safety of neoadjuvant immunotherapy combined with chemoradiotherapy or chemotherapy in esophageal cancer: A systematic review and meta-analysis. Front Immunol 2023;14:1117448. [Crossref] [PubMed]
- Gao L, Lu J, Zhang P, et al. Toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a single-center, single-arm clinical trial (ESONICT-2). J Gastrointest Oncol 2022;13:478-87. [Crossref] [PubMed]
- Li C, Zhao S, Zheng Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 2021;144:232-41. [Crossref] [PubMed]
- Clinical Guidelines Committee of Chinese College of Interventionalists. Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma (2023 edition). Zhonghua Yi Xue Za Zhi 2023;103:2674-94. [PubMed]
- Feng J, Zhao Y, Zhai L, et al. Efficacy and safety of transarterial chemoembolization combined with targeted therapy and immunotherapy versus with targeted monotherapy in unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2024;103:e38037. [Crossref] [PubMed]
- Liu Y, Qian L, Ma K, et al. Evaluation of clinical efficacy after combinational treatment of esophageal cancer using target artery perfusion of verapamil and chemotherapy. Transl Cancer Res 2017;6:1226-35. [Crossref]
- Yin MP, Xie PF, Zhao Y, et al. Clinical Evaluation of Transarterial Infusion Chemotherapy for Advanced Esophageal Cancer. J Cancer 2021;12:1493-8. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Tang R, Chen GF, Jin K, et al. Efficacy of continuous gastric artery infusion chemotherapy in relieving digestive obstruction in advanced gastric cancer. World J Gastrointest Oncol 2023;15:1283-94. [Crossref] [PubMed]
- Liu S, Xu K, Min J, et al. Effect of transarterial chemotherapy on the outcome and prognosis of patients with locally advanced proximal gastric cancer. Am J Transl Res 2023;15:1309-17. [PubMed]
- Li C, Yu P, Li H, et al. Study on the efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy regimen for III-IVA esophageal squamous cell carcinoma post-surgery. J Cardiothorac Surg 2024;19:26. [Crossref] [PubMed]
- Yu YK, Meng FY, Wei XF, et al. Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2024;168:417-428.e3. [Crossref] [PubMed]
- Verschoor YL, van de Haar J, van den Berg JG, et al. Neoadjuvant atezolizumab plus chemotherapy in gastric and gastroesophageal junction adenocarcinoma: the phase 2 PANDA trial. Nat Med 2024;30:519-30. [Crossref] [PubMed]
- Ding C, Guo Y, Zhou Y, et al. Perioperative tislelizumab plus chemotherapy for locally advanced resectable thoracic esophageal squamous cell carcinoma trial: a prospective single-arm, phase II study (PILOT trial). BMC Cancer 2023;23:1237. [Crossref] [PubMed]
- Park S, Lee Y, Lee J, et al. Neoadjuvant Nivolumab Therapy for Esophageal Squamous Cell Carcinoma: A Single-Arm, Phase II Study. Cancer Res Treat 2024;56:567-79. [Crossref] [PubMed]
- Luo RJ, Li ZJ, He ZF, et al. The efficacy and feasibility of neoadjuvant immunotherapy plus chemotherapy followed by McKeown minimally invasive oesophagectomy for locally advanced oesophageal squamous cell carcinoma. J Minim Access Surg 2024;20:334-41. [Crossref] [PubMed]
- Zhao J, Hao S, Tian J, et al. Comparison of Neoadjuvant Immunotherapy Plus Chemotherapy versus Neoadjuvant Chemoradiotherapy for Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score Matching Study. J Inflamm Res 2023;16:3351-63. [Crossref] [PubMed]
- Zhang H, Zhang Z, Yang L, et al. Perioperative outcomes of neoadjuvant immunotherapy plus chemotherapy and neoadjuvant chemoradiotherapy in the treatment of locally advanced esophageal squamous cell carcinoma: a retrospective comparative cohort study. J Thorac Dis 2023;15:1279-88. [Crossref] [PubMed]
- Ma R, Yuan D, Mo C, et al. Factors affecting the ORR after neoadjuvant therapy of TP regimen combined with PD-1 inhibitors for esophageal cancer. Sci Rep 2023;13:6080. [Crossref] [PubMed]
- Wang H, Jiang Z, Wang Q, et al. Pathological response and prognostic factors of neoadjuvant PD-1 blockade combined with chemotherapy in resectable oesophageal squamous cell carcinoma. Eur J Cancer 2023;186:196-210. [Crossref] [PubMed]
- Yin J, Lin S, Fang Y, et al. Neoadjuvant therapy with immunoagent (nivolumab) or placebo plus chemotherapy followed by surgery and adjuvant treatment in subjects with resectable esophageal squamous cell carcinoma: study protocol of a randomized, multicenter, double blind, phase II trial (NATION-2203 trial). J Thorac Dis 2023;15:718-30. [Crossref] [PubMed]
- Yang Y, Li H, Chen X, et al. Comparison of neoadjuvant nab-paclitaxel plus immunotherapy versus paclitaxel plus immunotherapy for esophageal squamous cell carcinoma. Thorac Cancer 2023;14:700-8. [Crossref] [PubMed]


