
Machine learning-based model for the prediction of acute kidney injury following coronary artery bypass graft surgery in elderly Chinese patients
Highlight box
Key findings
• Prediction models for assessing the risk of acute kidney injury (AKI) after coronary artery bypass graft (CABG) were developed using nine machine-learning algorithms. Among these algorithms, the random forest model showed the best predictive ability in elderly patients. It had an area under the curve of the receiver operating characteristic curve of 0.737, and a 95% confidence interval of 0.687–0.784.
What is known, and what is new?
• AKI after CABG is a common and critical complication with high mortality rates. Previous studies have identified risk factors for AKI after CABG, including body mass index, hypertension, cardiopulmonary bypass (CPB) duration, and postoperative low cardiac output syndrome.
• The risk factors identified in this study differed to those identified in previous research, showing the complexity of factors affecting AKI after CABG. Unlike previous studies in which various models showed superiority in different scenarios, in this study, the RF model had the optimal predictive efficacy.
What is the implication, and what should change now?
• This RF model showed good predictive performance and could be helpful in the early detection of elderly Chinese patients at high risk of AKI post-CABG surgery. Clinicians could use this model to enhance intraoperative and postoperative monitoring, reduce the use of nephrotoxic drugs, and implement early intervention strategies to decrease the incidence of AKI and postoperative mortality. Future prospective research needs to be conducted to further validate the effectiveness of the model in clinical settings.
Introduction
With the aging global population, the incidence of coronary artery disease (CAD) continues to increase (1). Coronary artery bypass graft (CABG) significantly reduces mortality in CAD patients (2). However, acute kidney injury (AKI), which is characterized by rapid renal function decline post-surgery, is a critical complication of CABG (3,4), and occurs in up to 38% of CABG patients, 3% of whom require dialysis (5). Moreover, one-fifth of AKI patients progress to chronic kidney disease (CKD) within 90 days; the 90-day mortality rate can be as high as 43.5% and long-term mortality rate ranges from 11.8% to 29.8% (6,7).
Age is a significant risk factor for AKI following CABG, such that elderly patients face a higher risk due to decreased renal function. However, current research on AKI in elderly CABG patients remains limited. These patients often experience extended hospital stays, greater treatment challenges, and financial burdens, as well as an increased incidence of dialysis and reduced postoperative survival rates (7). Thus, it is of great clinical significance to establish a risk prediction model for postoperative AKI and identify elderly high-risk patients.
This study sought to retrospectively analyze the data of elderly patients undergoing CABG at Beijing Anzhen Hospital, and use nine machine-learning models to identify high-risk patients for AKI based on preoperative and intraoperative variables. The study will facilitate enhanced intraoperative and postoperative monitoring, and provide robust support for optimizing the treatment strategies of elderly patients at high risk of AKI. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-264/rc).
Methods
Study population
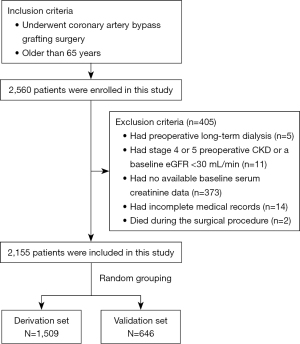
Patients admitted to the Beijing Anzhen Hospital from January 2019 and December 2020 were enrolled in this retrospective study. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: be aged 65 years or older; and have undergone isolated CABG surgery without additional procedures. Patients were excluded from the study if they met any of the following exclusion criteria: had preoperative long-term dialysis; had a history of kidney transplantation; had stage 4 or 5 preoperative CKD; had a baseline estimated glomerular filtration rate (eGFR) <30 mL/min; had a preoperative diagnosis of AKI; died during the surgical procedure; had no available baseline serum creatinine (SCr) data; had incomplete medical records; and/or had postoperative hospitalization lasting more than 90 days. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2021-069), and patient privacy was maintained throughout the study. All the patients signed an informed consent form prior to recruitment.
Definition of postoperative AKI after CABG
Postoperative AKI after CABG was defined according to the Kidney Disease Improvement Global Outcomes criteria (8). In general, postoperative AKI was characterized by either a SCr increase of at least 50% from the baseline within 7 days of the surgery, or a SCr increase of 26.5 µmol/L within 48 hours of the surgery. Under the AKI severity classification, stage 1 was defined as a SCr level 1.5–1.9 times that of the baseline value, or an increase ≥26.5 µmol/L; stage 2 was defined as a SCr level 2.0–2.9 times that of the baseline; and stage 3 was defined as a SCr level ≥3.0 times that of the baseline, or ≥353.6 µmol/L, or the initiation of renal replacement therapy. Due to the unavailability of detailed perioperative urine output data, urine output was not considered in the assessment of renal function. Baseline SCr was measured within 7 days before the surgical procedure.
Statistical analysis
All the statistical analyses were conducted using Python (version 3.5, http://www.python.org). To enhance the performance of the prediction models, the following nine distinct supervised machine-learning algorithms for model development and evaluation were employed: logistic regression (LR), which estimates probabilities by maximizing the log-likelihood; simple decision tree (DT), which performs categorical prediction based on tree-structured features; random forest (RF), which improves generalization through ensemble learning; support vector machine (SVM), which maximizes class separation margins; extreme gradient boosting (XGBoost) and gradient boosting, which progressively refine predictions through boosting techniques; light gradient boosting machine (lightGBM), which is an efficient and scalable gradient boosting implementation; K-nearest neighbor (KNN), which makes predictions by neighborhood voting; and adaptive boosting (AdaBoost), which iteratively focuses on misclassified instances and combines weak classifiers into a strong one. These machine-learning algorithms allowed us to develop and evaluate various predictive models to determine the model with the most efficient predictive performance.
The entire dataset was randomly divided, such that 70% of the dataset was allocated to the derivation set, and 30% of the dataset was allocated to the validation set. For variables, missing values were replaced using the mean value. Variables with over 20% missing data were excluded from the subsequent analysis, as a large amount of missing data can introduce bias and affect the reliability of an analysis. The continuous variables were normalized when included in the models to enable variables with different units of measurement to be compared and analyzed equally.
During the model training and optimization, the five-fold cross-validation technique was used to determine the optimal parameters for each model, effectively minimizing the risk of overfitting. SHapley Additive exPlanations (SHAP) values were applied for feature importance ranking. SHAP, which is grounded in game theory, offers insights into how individual features influence the predictions of machine-learning models. The SHAP values facilitated our understanding of how each feature contributed to individual predictions made by the model and was also used to evaluate the importance of features.
To assess the predictive performance and accuracy of different machine-learning models, we plotted receiver operating characteristic (ROC) curves and calculated and compared the areas under the curves (AUCs) of the ROCs to analyze the ability of the various machine-learning models to predict AKI risk. Ultimately, we evaluated the performance of each machine-learning model by computing and comparing the following metrics: accuracy, precision, recall, F1-score, and AUC.
Results
Patient characteristics
The study included 2,155 participants, who had a median age of 69.2 years. The patients were further categorized based on the postoperative AKI definition. Of the patients, 294 (13.6%) had stage 1 AKI, 52 (2.4%) had stage 2 AKI, and 19 (0.9%) had stage 3 AKI. A comprehensive summary of the baseline demographic and clinical characteristics of the patients is provided in Table 1. Males accounted for 68.4% of the cohort. Among the comorbidities, hypertension was the most prevalent comorbidity, affecting 65.7% of the patients, followed closely by hyperlipidemia (54.8%) and type 2 diabetes mellitus, which was present in 37.7% of the patients. The patients were randomly stratified into two separate datasets: the derivation dataset, which comprised 1,509 patients, and the validation dataset, which comprised 646 patients. The process of cohort selection is depicted in Figure 1.
Table 1
| Variables | Values (N=2,155) |
|---|---|
| Demographics | |
| Age (years)† | 69.2 (6.0) |
| Male sex | 1,473 (68.4) |
| Smoker | 436 (20.2) |
| Drinker | 343 (15.9) |
| Body mass index (kg/m2)† | 25.2 (4.1) |
| Complication | |
| Hypertension | 1,416 (65.7) |
| Diabetes mellitus | 812 (37.7) |
| Hyperlipemia | 1,181 (54.8) |
| Prior MI | 307 (14.2) |
| Prior cerebral infarction | 250 (11.6) |
| Prior PCI | 230 (10.7) |
| Prior CABG | 37 (1.7) |
| NYHA functional class | |
| Class III/IV | 431 (20.0) |
| Vital signs at admission† | |
| Heart rate (bpm) | 76 (12.0) |
| Systolic blood pressure (mmHg) | 130.0 (18.0) |
| Diastolic blood pressure (mmHg) | 76.0 (12.0) |
| Mean arterial pressure (mmHg) | 94.7 (11.7) |
| Laboratory examinations at admission† | |
| eGFR (mL/min) | 77.4 (27.6) |
| Serum creatinine (μmol/L) | 72.1 (22.0) |
| UA (μmol/L) | 317.9 (123.3) |
| BNP (pg/mL) | 164.0 (250.0) |
| PLT count (×109/L) | 206 (81.0) |
| Low-density lipoprotein cholesterol (mmol/L) | 2.22 (1.00) |
| Triglycerides (mmol/L) | 1.31 (0.80) |
| Total cholesterol (mmol/L) | 3.79 (1.20) |
| High-density lipoprotein cholesterol (mmol/L) | 1.00 (0.30) |
| ALT (U/L) | 20.0 (15.0) |
| AST (U/L) | 21.0 (11.0) |
| Preoperative concomitant medication | |
| Aspirin | 566 (26.3) |
| ACE inhibitor/ARB | 347 (16.1) |
| Beta blocker | 1,651 (76.6) |
| Statin therapy | 402 (18.7) |
| PPI | 533 (24.7) |
| Loop diuretic | 432 (20.0) |
| Thiazide | 83 (3.9) |
| Spirolactone | 232 (10.8) |
| Contrast agent | 549 (25.5) |
| Metformin | 219 (10.2) |
| Intraoperative | |
| RBC transfusion | 502 (23.3) |
| PLT transfusion | 36 (1.7) |
| Plasma transfusion | 194 (9.0) |
| Use of IABP | 161 (7.5) |
| Use of ECMO | 7 (0.3) |
| Use of CPB | 359 (16.7) |
| Use of epinephrine | 501 (23.2) |
| Use of norepinephrine | 1,216 (56.4) |
| Use of isoprenaline | 126 (5.8) |
| Use of dopamine | 1,836 (85.2) |
| Use of cephalosporin | 1,781 (82.6) |
| Operation† | |
| Operation time (h) | 4 (1.0) |
| Urine output (×100 mL) | 12 (12.0) |
| Bleeding volume (×100 mL) | 8 (4.0) |
| Total liquid intake (×100 mL) | 25.0 (9.5) |
| Washed RBC transfusion volume (×100 mL) | 2.4 (4.0) |
†, the continuous data are expressed as the median (interquartile range) and were calculated using the Mann-Whitney U-test. The categorical data are expressed as the count (percentage), and were calculated using the Chi-squared test. ACE, angiotensin-converting enzyme; AKI, acute kidney injury; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump; LVED, left ventricular end-diastolic diameter; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PLT, platelet; PPI, proton pump inhibitor; RBC, red blood cell; UA, uric acid.
Establishment and validation of machine-learning models
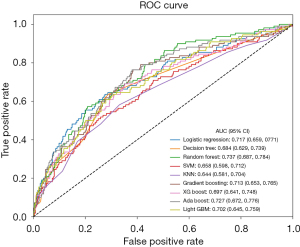
To predict the postoperative AKI events, the following machine-learning methods were used with qualified variables as the input variables: LR, DT, RF, SVM, XGboost, AdaBoost, gradient boosting, lightGBM, and KNN. Table 2 and Figure 2 present the performance of the nine machine-learning models employed in this study, providing a clear comparison of their predictive capabilities. The RF model had the highest AUC [0.737, 95% confidence interval (CI): 0.687–0.784], while the KNN model had the lowest AUC (0.644, 95% CI: 0.581–0.704).
Table 2
| Model | Accuracy | Precision | Recall | F1-score | AUC |
|---|---|---|---|---|---|
| Random forest | 0.830 | 0.000 | 0.000 | 0.000 | 0.737 |
| AdaBoost | 0.833 | 1.000 | 0.018 | 0.036 | 0.727 |
| Logistic regression | 0.836 | 0.591 | 0.118 | 0.197 | 0.717 |
| Gradient boosting | 0.838 | 0.727 | 0.073 | 0.132 | 0.713 |
| LightGBM | 0.832 | 0.600 | 0.027 | 0.052 | 0.702 |
| XGBoost | 0.832 | 0.556 | 0.046 | 0.084 | 0.697 |
| Decision tree | 0.821 | 0.406 | 0.118 | 0.183 | 0.684 |
| SVM | 0.830 | 0.000 | 0.000 | 0.000 | 0.658 |
| KNN | 0.830 | 0.000 | 0.000 | 0.000 | 0.644 |
AdaBoost, adaptive boosting; AUC, area under the curve; KNN, K-nearest neighbors; LightGBM, light gradient boosting machine; SVM, support vector machine; XGBoost, extreme gradient boosting.
To identify the features that had the greatest effect on the prediction model, a SHAP summary diagram of the RF model was plotted (Figure 3A), and the top 20 features relevant to the prediction model were identified. This plot illustrates the relationship between the feature values and their SHAP values in the training dataset, where a higher SHAP value for a feature indicates a greater likelihood of AKI occurrence according to the predictive model.
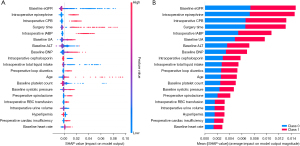
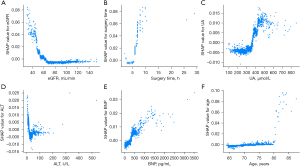
Additionally, a SHAP dependence plot (Figure 3B) was used to gain insights into how individual features affect the output of the RF predictive model. In the SHAP dependence visualization, the vertical dimension (y-axis) represents the SHAP values of features, while the horizontal dimension (x-axis) represents the feature values. This shows how the significance of a particular feature fluctuates in accordance with its varying values across different scenarios. A SHAP value that exceeds zero for a specific feature indicates an elevated risk of AKI development. Figure 4 shows the effects of age, the eGFR, surgery time, uric acid (UA), alanine aminotransferase (ALT), and B-type natriuretic peptide (BNP) on SHAP values, and SHAP values represent their effect on AKI outcomes.
Discussion
This study sought to predict the risk of AKI in elderly patients after CABG surgery. Our risk prediction model can be used to estimate the likelihood of AKI after CABG, which could help clinicians to identify patients at high risk of AKI, and implement appropriate clinical prevention measures. Unlike many previous studies, we specifically focused on elderly patients (9,10). We tested nine machine-learning models to predict AKI risk in this population, and found that the RF model outperformed all the other models. To better understand the factors driving the predictions of the model, SHAP was used to interpret the importance of different variables in the model.
Due to remarkable advantages related to accuracy, intelligence, and sensitivity, there has been an increased adoption of machine-learning models for disease prediction in the medical field (9,11). A study conducted by Song et al. indicated that gradient boosting DT models showed superior performance in predicting the risk of AKI following off-pump CABG (12). Additionally, Wang et al. showed that the lightGBM model surpassed other models in terms of predictive performance (13). Conversely, Tseng et al. showed that, among the single models, the RF model showed the best performance, while among the ensemble models, the RF + XGboost combination model had the highest AUC value, which surpassed that of the single RF model (10).
However, the results of the present study were not entirely consistent with these findings. Our results revealed that the RF model exhibited optimal predictive efficacy. The RF model possesses significant advantages in processing extensive electronic medical record data, efficiently managing a large number of input variables, and adapting to different magnitudes of data. As an extension of traditional DT classifiers, the RF model reduces the limitations of DT, thereby enhancing its predictive accuracy and reliability (14).
The occurrence of AKI following cardiac surgery is multifactorial, encompassing a variety of mechanisms. These include acute glomerular necrosis, prolonged hospital stay, microembolism, neurohormonal activation, the presence of exogenous and endogenous toxins, metabolic disturbances, hemodynamic alterations, inflammatory responses, ischemia-reperfusion injury, oxidative stress, and the administration of red blood cell transfusions. These factors play a role in the development and progression of AKI after cardiac surgery (3,15-17).
Yue et al. reported that factors such as age, body mass index (BMI), hypertension, eGFR, cardiopulmonary bypass (CPB) duration, and postoperative low cardiac output syndrome are significant independent risk factors for AKI following CABG (18). In a study by Li et al., several factors such as advanced age, an elevated BMI, a decreased baseline eGFR, low ejection fraction, statin administration, red blood cell transfusions, adrenaline usage, the implementation of an intra-aortic balloon pump (IABP), the postoperative syndrome of low cardiac output, and the need for reoperation due to bleeding were independent predictors of AKI (19). Our findings showed some inconsistencies with the risk factors reported in previous studies (18,19). Notably, our study found that various factors contribute to the risk of AKI following surgery, including a low baseline eGFR, low PLT, advanced age, elevated levels of UA and BNP, prolonged surgical duration, and the use of an IABP. Further, the effect of preoperative and intraoperative medications on the incidence of postoperative AKI is significant and cannot be ignored. The preoperative administration of contrast agents or nephrotoxic drugs, such as diuretic and antibiotics, has been shown to potentially cause renal tubular obstruction, leading to subtle renal damage that may not be detectable through changes in eGFR (20).
The clinical significance of this study is that our model can predict the risk of postoperative AKI in elderly patients. Although preoperative risk factors cannot be completely avoided and a single treatment strategy may not suffice due to complex pathogenesis, effective preoperative prediction can be used to assess the risk of each elderly patient. Patients classified as low-risk would continue standard perioperative care regimen with routine monitoring. Moderate-risk patients should strengthen renal function monitoring, particularly to avoid the use of nephrotoxic drugs. High-risk cases would trigger multidisciplinary team consultations to develop individualized care plans. These plans would integrate preoperative surgical optimization, intraoperative renal protective strategies, and contingency plans for early postoperative renal replacement therapy, allowing for the earlier implementation of effective intervention strategies to reduce the incidence of AKI and postoperative mortality after CABG surgery.
This study had some limitations. First, this study was conducted as a single-center investigation, inherently limited by the demographic characteristics of patients, medical resources, and clinical practice patterns specific to our center. Consequently, the research findings may not be directly generalizable to other centers. Variability exists among patient populations across different centers in terms of genetic backgrounds, comorbidity profiles, and surgical protocols, which may lead to fluctuations in the model’s predictive performance when applied to diverse cohorts. To enhance the universality of our findings, subsequent studies will actively establish multi-center collaborations to incorporate broader patient datasets, re-evaluate the model’s validity and stability, and strengthen the general applicability of the model. Second, retrospective study designs inevitably carry risks of selection bias and information bias. In this study, despite implementing stringent inclusion and exclusion criteria for patient selection and meticulously verifying data to minimize information bias, these measures could not entirely eliminate the impact of residual biases. Therefore, we plan to conduct a prospective study to validate the models and findings from this research, enabling more precise evaluation of the associations between preoperative/intraoperative risk factors and postoperative AKI incidence. Third, this study only utilized an internal validation set for model evaluation without conducting external validation. We plan to actively seek appropriate external datasets for external validation. By comparing the model’s performance metrics across diverse datasets, we aim to further refine the model evaluation framework. Forth, our study included only available risk factors, omitting variables with more than 20% missing data or overlooked variables, which could have affected the predictive accuracy of the model. Finally, the predictive capability of the model was assessed using ROC values in both the derivation and validation sets. Future prospective research is required to determine whether the implementation of this predictive model can reduce the risk of AKI in clinical settings.
Conclusions
This study sought to establish an optimal machine-learning model to predict AKI in elderly patients after CABG. This model has important implications for clinical practice and provides a new approach for improving the prognosis of these patients. Based on preoperative and intraoperative variables, nine machine-learning models were used to identify patients at high risk of AKI. Among all the models examined, the RF model had the best predictive performance. The RF model could assist clinicians to predict and reduce the occurrence of AKI following CABG.
Acknowledgments
We would like to thank all the participants included in this study.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-264/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-264/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-264/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-264/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2021-069). All the patients signed an informed consent form prior to recruitment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xi Z, Qiu H, Guo T, et al. Prevalence, Predictors, and Impact of Coronary Artery Ectasia in Patients With Atherosclerotic Heart Disease. Angiology 2023;74:47-54. [Crossref] [PubMed]
- Wintgen L, Dakkak AR, Shakaki MA, et al. Acute kidney injury following coronary artery bypass grafting and control angiography: a comprehensive analysis of 221 patients. Heart Vessels 2021;36:1-6. [Crossref] [PubMed]
- Vives M, Hernandez A, Parramon F, et al. Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis 2019;12:153-66. [Crossref] [PubMed]
- Wang R, Wang X, Zhu Y, et al. Acute kidney injury following on-pump or off-pump coronary artery bypass grafting in elderly patients: a retrospective propensity score matching analysis. J Cardiothorac Surg 2020;15:186. [Crossref] [PubMed]
- Fisher LA, Stephenson S, Reid MT, et al. Acute kidney injury following cardiopulmonary bypass in Jamaica. JTCVS Open 2022;11:161-75. [Crossref] [PubMed]
- Rimes-Stigare C, Frumento P, Bottai M, et al. Long-term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care 2015;19:383. [Crossref] [PubMed]
- Fan X, Shao Z, Gao S, et al. Clinical characteristics and risk factors of cardiac surgery associated-acute kidney injury progressed to chronic kidney disease in adults: A retrospective, observational cohort study. Front Cardiovasc Med 2023;10:1108538. [Crossref] [PubMed]
- Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015;87:62-73. [Crossref] [PubMed]
- Lee HC, Yoon HK, Nam K, et al. Derivation and Validation of Machine Learning Approaches to Predict Acute Kidney Injury after Cardiac Surgery. J Clin Med 2018;7:322. [Crossref] [PubMed]
- Tseng PY, Chen YT, Wang CH, et al. Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit Care 2020;24:478. [Crossref] [PubMed]
- Fei Y, Hu J, Li WQ, et al. Artificial neural networks predict the incidence of portosplenomesenteric venous thrombosis in patients with acute pancreatitis. J Thromb Haemost 2017;15:439-45. [Crossref] [PubMed]
- Song Y, Zhai W, Ma S, et al. Machine learning-based prediction of off-pump coronary artery bypass grafting-associated acute kidney injury. J Thorac Dis 2024;16:4535-42. [Crossref] [PubMed]
- Wang X, Xu L, Guan C, et al. Machine learning-based risk prediction of acute kidney disease and hospital mortality in older patients. Front Med (Lausanne) 2024;11:1407354. [Crossref] [PubMed]
- Li K, Yu N, Li P, et al. Multi-label spacecraft electrical signal classification method based on DBN and random forest. PLoS One 2017;12:e0176614. [Crossref] [PubMed]
- Ejmalian A, Aghaei A, Nabavi S, et al. Prediction of Acute Kidney Injury After Cardiac Surgery Using Interpretable Machine Learning. Anesth Pain Med 2022;12:e127140. [PubMed]
- Jin L, Shan L, Yu K, et al. Postoperative acute kidney injury increases short- and long-term death risks in elderly patients (≥ 75 years old) undergoing coronary artery bypass graft surgery. Int Urol Nephrol 2024;56:1497-508. [Crossref] [PubMed]
- Maruniak S, Loskutov O, Swol J, et al. Factors associated with acute kidney injury after on-pump coronary artery bypass grafting. J Cardiothorac Surg 2024;19:598. [Crossref] [PubMed]
- Yue Z, Yan-Meng G, Ji-Zhuang L. Prediction model for acute kidney injury after coronary artery bypass grafting: a retrospective study. Int Urol Nephrol 2019;51:1605-11. [Crossref] [PubMed]
- Li Y, Hou XJ, Liu TS, et al. Risk factors for acute kidney injury following coronary artery bypass graft surgery in a Chinese population and development of a prediction model. J Geriatr Cardiol 2021;18:711-9. [PubMed]
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


