
Single venous cannulation for bi-atrial venoarterial extracorporeal membrane oxygenation in bridge to transplantation: a two-in-one approach
Highlight box
Surgical highlights
• A single-cannula strategy employing a multiperforated venous cannula, inserted transseptally and percutaneously for left atrial decompression during venoarterial extracorporeal membrane oxygenation (VA-ECMO), ensures left ventricular (LV) unloading by capitalizing on the intrinsic pressure gradient between the left atrium (LA), and the right-sided venous system.
What is conventional and what is novel/modified?
• Traditional LV unloading often employs dual-cannula strategies, intra-aortic balloon pumps, or microaxial pumps (Impella). These approaches can be effective but involve higher procedural complexity and additional arterial or venous access.
• This two in one approach ensure LA drainage and venous return into a single multiperforated cannula. The approach leverages real-time imaging (transesophageal echocardiography and fluoroscopy) to ensure accurate placement, simplifying the procedure.
What is the implication, and what should change now?
• Eliminating secondary venous or arterial access, the single-cannula strategy lowers the risk of complications such as bleeding, vascular injury, and infection. It provides a practical option for critically ill patients, where time and safety are paramount.
• Broader adoption of this technique could streamline LV unloading in VA-ECMO, offering a less invasive alternative to multi-device methods. Further studies with larger patient populations are needed to confirm its long-term benefits and optimize patient selection.
Introduction
Background
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is a critical and well-established intervention used in cases of severe cardiogenic shock (1,2), biventricular failure, or refractory heart failure. VA-ECMO provides a temporary external circuit that oxygenates the blood, bypassing the heart and lungs, to maintain systemic circulation and tissue perfusion. This approach is particularly useful in acute clinical scenarios as a bridge to recovery, heart transplantation, or long-term mechanical circulatory support.
One of the main challenges with VA-ECMO is the increase in the left ventricular (LV) afterload due to retrograde blood flow from the ECMO circuit into the aorta. This increases the resistance that the LV must overcome, leading to LV distension (3,4). If not adequately managed, LV distension can result in pulmonary congestion, myocardial ischemia, and delayed cardiac recovery. Therefore, efficient LV unloading strategies are essential in patients undergoing VA-ECMO (5).
Rationale
LV unloading during VA-ECMO is essential to reduce pulmonary edema, improve oxygenation, and support myocardial recovery. Conventional strategies for LV decompression, such as intra-aortic balloon pumps (IABPs) and Impella devices, are widely used but involve significant procedural complexities and risks, including bleeding, vascular injury, and infection. These methods often require additional arterial access, which further increases procedural complexity (6,7).
In studies describing percutaneous left atrium (LA) unloading (3,8), a two-cannula technique is frequently employed during VA-ECMO. This approach typically uses a primary venous ECMO cannula to drain blood from the femoral vein, providing circulatory support, while a secondary transseptal LA drainage cannula is inserted via contralateral venous access and placed transseptally to decompress the LA (3).
The LA drainage cannula, which was integrated into the ECMO venous circuit, functioned as the secondary cannula. This two-cannula configuration effectively unloads the LV by draining blood from the LA, reducing LV pressure, and preventing complications, such as LV distension and pulmonary edema (3).
A less invasive alternative involves transseptal left atrial decompression using a single venous multistage drainage cannula (6). A Bio-Medicus NextGen cannula (Medtronic, Mineapolis, USA), commonly used in VA-ECMO procedures, was used. It is equipped with six large-tip side holes to facilitate optimal drainage, with perforations along a 25 cm segment. This configuration enables both venous drainage and left atrial decompression via transseptal puncture, thereby eliminating the need for additional venous cannulation.
Objective
This study aimed to assess the feasibility, safety, and clinical outcomes of employing a single multistage venous drainage cannula via a transseptal approach for left atrial decompression in patients receiving VA-ECMO for biventricular cardiogenic shock. Additionally, we sought to validate previously reported data from case reports (8), specifically regarding the safety of transseptal cannulation and ECMO flow achieved by using a single-cannula configuration. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1993/rc).
Preoperative preparations and requirements
Patient selection (Table 1)
Table 1
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age (years)/sex | 48/M | 54/M | 49/M | 42/M |
| Cardiac shock etiology | Hypertrophic cardiomyopathy | Ischemic cardiomyopathy | Dilated cardiomyopathy | Dilated cardiomyopathy |
| Diabetes mellitus | No | No | No | No |
| BMI (kg/m2) | 23.0 | 22.5 | 26.2 | 26.4 |
| Coronary artery disease | No | Yes | No | No |
| Echocardiographic parameters | ||||
| LVEF (%) | 13 | 22 | 25 | 11 |
| LA sludge | Yes | Yes | Yes | Yes |
| E/A ratio | >2 | – | >2 | – |
| LVED diameter (mm) | 69 | – | 60 | – |
| Mitral insufficiency | III | IV | II | III |
| PA catheterization | ||||
| PAD (mmHg) | 54 | 32 | 65 | 53 |
| PAS (mmHg) | 85 | 54 | 91 | 85 |
| PAM (mmHg) | 64 | 43 | 74 | 61 |
| Biochem parameters | ||||
| Lac (mmol/L) | 5.5 | – | 2.39 | 1.52 |
| Trop (ng/L) | 31 | 710 | 42 | 52 |
| NT-proBNP (ng/L) | 6,316 | 17,009 | 19,933 | 22,428 |
| GGT (U/L) | 171 | 113 | 247 | 128 |
| AST (U/L) | 578 | 30 | 1,316 | 48 |
| ALT (U/L) | 491 | 51 | 1,015 | 127 |
| Cr (µmol/L) | 137 | 475 | 118 | 117 |
Mitral insufficiency: severity of mitral regurgitation. ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; Cr, creatinine; E/A ratio, early to late ventricular filling velocity ratio; GGT, gamma-glutamyl transferase; Lac, lactate level; LA sludge, dense spontaneous echo contrast in the left atrium; LVED, left ventricular end-diastolic; LVEF, left ventricular ejection fraction; M, male; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAD, pulmonary artery diastolic pressure; PAM, pulmonary artery mean pressure; PAS, pulmonary artery systolic pressure; Trop, troponin; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Four male patients, all candidates for heart transplantation, were admitted to the cardiac intensive care unit (ICU) with severe biventricular decompensation refractory to maximal inotropic therapy. Echocardiographic evaluation revealed severe LV dysfunction (ejection fractions ranging from 11% to 25%) and dense spontaneous echo contrast (“sludge”) in the LA, indicating significant stasis and a high thromboembolic risk. Hemodynamic instability was compounded by elevated pulmonary artery pressures, with diastolic pressures ranging from 32 to 65 mmHg, and clinical signs of progressive pulmonary congestion.
The biological parameters reflect the severity of the conditions. Elevated lactate levels (ranging from 1.52 to 5.5 mmol/L) signified ongoing tissue hypoperfusion. High NT-proBNP levels, exceeding 6,000 ng/L in all cases and peaking at 22,428 ng/L, indicate severe myocardial strain. Markers of hepatic injury, such as elevated transaminase levels [aspartate transaminase (AST) ranging from 30 to 1,316 U/L and alanine transaminase (ALT) ranging from 51 to 1,015 U/L], were present, consistent with congestive hepatopathy. Given the worsening clinical status and persistent pulmonary edema, a multidisciplinary heart team consultation in the ICU determined that transseptal left atrial decompression was required to mitigate left atrial pressure and reduce pulmonary congestion.
Operative environment
All procedures were conducted in a hybrid operating room equipped with fluoroscopy and transesophageal echocardiography (TEE) to provide real-time imaging. The hybrid operating room configuration facilitated optimal visualization during transseptal puncture and cannula placement, thereby significantly reducing the risks associated with suboptimal positioning or complications, such as atrial perforation. Using TEE alongside fluoroscopy ensures precise cannula placement and mitigates the risk of compromising the integrity of the surrounding structures, including the fragile left atrial appendage and pulmonary veins (9).
Step-by-step description (Figure 1)
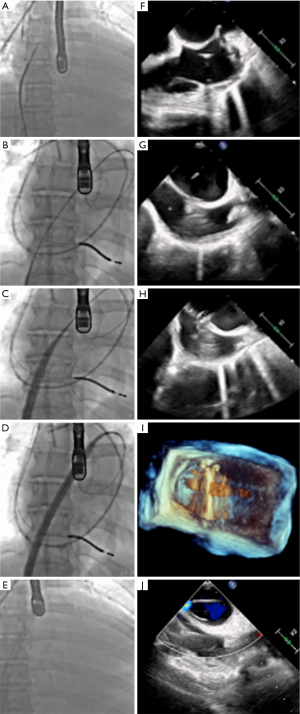
Femoral venous and arterial access and cannulation (Video 1)
A weight-based heparin bolus was administered prior to cannulation. Activated clotting time (ACT) was monitored throughout the procedure, and an intravenous heparin bolus was administered to the target ACT >250 s before and after the transseptal puncture.
For arterial percutaneous cannulation, an ultrasound-guided percutaneous insertion technique utilizing serial dilation via the Seldinger technique has been employed. Guidewire positioning was subsequently verified using fluoroscopy.
For venous femoral cannulation, the right groin was selected as the site for the transseptal puncture. Ultrasound-guided percutaneous insertion was performed via serial dilation (Seldinger technique).
Transseptal puncture and balloon atrioseptostomy (Video 2)
The VersaCross steerable system (Baylis Medical, Montreal, Canada) was used to perform transseptal puncture.
The J-tipped guidewire positioning was confirmed by fluoroscopic examination. An 8.5F TorFlex transseptal guiding sheath and dilator (Baylis Medical) were advanced to the superior vena cava using TTE guidance (Figure 1A,1F). The guidewire was exchanged for an RF NRG Transseptal needle (Baylis Medical), and the entire assembly was maneuvered to the transseptal puncture location under real-time TEE guidance. A 1-second radiofrequency pulse was delivered using an RFP-100A generator (Baylis Medical). After LA access was obtained, a stiff ProTrack Pigtail wire (Baylis Medical) was positioned in the middle LA (Figure 1B,1G), ensuring preservation of the surrounding structures, with particular attention paid to the left atrial appendage.
Atrial balloon septostomy was performed with a 10-mm balloon (Figure 2A,2B) to facilitate advancement of the 25 F Bio-Medicus NextGen cannula, minimizing the risk of atrial perforation and trauma to the surrounding cardiac structures.

Bio-Medicus NextGen cannula placement (Video 3)
The Bio-Medicus NextGen cannula was advanced over the guidewire into the LA (Figure 1C,1D,1H,1I). Its multiple side holes facilitate simultaneous drainage from the inferior vena cava (IVC) and LA, ensuring effective venous return and left atrial decompression. The proximal portion of the cannula remained in the IVC, while the distal tip was positioned in the LA. TEE imaging confirmed the accurate placement of the cannula (Figure 1E,1J), ensuring that it did not interfere with critical structures, such as the mitral valve. Upon proper positioning of the cannula, it was connected to the VA-ECMO circuit to initiate circulatory support and LV unloading (Figure 3A,3B). A comprehensive visualization of transseptal VA-ECMO cannulation is provided (Figure 4). Figure 4A features a three-dimensional computed tomography (CT) reconstruction, detailing the anatomical pathway of the femoral venous cannula. The cannula is seen advancing transthoracically through the IVC (d), entering the right atrium (c), crossing the interatrial septum (b), and ultimately reaching the LA (a). Figure 4B complements this with a chest radiograph of the same patient, showcasing the positioning of the venous multistage cannula within the thoracic cavity. These imaging modalities together ensure accurate cannula placement and effective monitoring during ECMO support.


Postoperative considerations and tasks (Table 1)
Monitoring and follow-up
Postoperatively, the patients were closely monitored to evaluate the efficacy of LV unloading and resolution of pulmonary congestion. TEE and chest radiography were used to confirm appropriate cannula placement and assess procedural outcomes. Daily TEE examinations were performed to evaluate LV distension, providing critical insights into the efficacy of decompression and unloading. Improvements in systemic oxygenation and reductions in LV pressure are regarded as key indicators of procedural success, reflecting effective hemodynamic stabilization and LV unloading.
Complications and safety (Table 2)
Table 2
| Parameter | Patient 1 (48 years/M) |
Patient 2 (54 years/M) |
Patient 3 (49 years/M) |
Patient 4 (42 years/M) |
|---|---|---|---|---|
| Venous cannulae size (Fr) | 25 | 25 | 25 | 25 |
| BSA (m2) | 1.82 | 1.94 | 2.08 | 1.77 |
| Bleeding, stroke or tamponnade | No | No | No | No |
| Mean ECMO flow (L/min) | 5.0 | 3.5 | 4.7 | 3.8 |
| SVO2 (%) | 78 | 73 | 65 | 70 |
| ECMO duration (days) | 9 | 7 | 4 | 5 |
| Survival | Discharged | Deceased | Discharged | Discharged |
| Post-ECMO | Tx | MOF | Tx | Tx |
BSA, body surface area; ECMO, extracorporeal membrane oxygenation; M, male; MOF, multiorgan failure; SVO2, mixed venous oxygen saturation; Tx, transplant; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
No major procedural complications such as bleeding, cardiac tamponade, or cerebrovascular accident were observed during or after transseptal cannulation. All four patients exhibited significant improvement in attaining overall hemodynamic stability. Moreover, the intervention facilitated enhanced pulmonary oxygenation, as evidenced by improved mixed venous oxygen saturation (SVO2) values across the cohort. The absence of severe complications underscores the procedural safety and clinical efficacy of transseptal cannulation in VA-ECMO for LV unloading.
Tips and pearls
Imaging guidance: continuous utilization of TEE and fluoroscopic guidance are essential for performing a transseptal puncture and confirming precise cannula placement. Real-time imaging mitigates the risk of atrial or pericardial perforation, with particular emphasis on left atrial appendage perforations (9,10).
Single-cannula efficiency: the utilization of a single multistage perforated cannula for venous drainage and LV unloading simplifies the procedure, thereby reducing the need for additional arterial access and enhancing safety.
Discussion
Surgical highlights
Our findings demonstrated the feasibility and safety of LV unloading using a multi-staged venoarterial drainage cannula, the Bio-Medicus NextGen cannula (Medtronic), through a transseptal approach in patients undergoing VA-ECMO. This single-cannula strategy simplifies the procedure by avoiding the need for additional arterial access (Figure 1), in contrast to traditional methods such as the IABP or Impella device. The primary advantage of this approach is its reduction in procedural complexity, which translates to a decreased risk of complications such as bleeding, vascular injury, and infection, which are complications typically associated with arterial cannulation (3,5,7). This method offers a potentially less invasive and safer alternative, particularly in critically ill patients (1,8,11).
An important consideration in VA-ECMO is that it does not directly decompress the LV, which can lead to blood accumulation, increased LV end-diastolic pressure (LVEDP), and potentially severe pulmonary edema if the heart cannot adequately eject blood (8,12). Signs of LV distension include dyspnea, increased tracheal secretions, and pulmonary congestion. Echocardiography is invaluable for diagnosing LV distension by assessing valve regurgitation and LV dimensions (3).
Mechanical decompression of the left heart is necessary if the inotropes fail to relieve LV distension. Several methods are available for this purpose, including percutaneous transseptal LA venting, balloon atrial septostomy, and surgical venting through the right upper pulmonary vein or transapical LV cannulation. Each of these strategies has distinct advantages depending on the clinical scenario, and integrating LV venting is crucial for improving outcomes, particularly in patients with a high LVEDP (11,13,14).
The present study focused on demonstrating the feasibility and safety of a single-cannula transseptal approach for left atrial decompression during VA-ECMO (5). This approach leverages the natural pressure gradient between the left and right atria to facilitate effective decompression using a multiperforated venous cannula (Bio-Medicus NextGen, Medtronic). The rationale behind this design is to simplify the procedure, reduce procedural complexity, and mitigate the risks associated with dual-cannula strategies, such as atrial wall suction or mechanical damage to cardiac structures.
Previous studies have highlighted the challenges of directly measuring the left atrial pressure and flow in such setups, particularly when using multiperforated cannulas (5,15). Furthermore, although dual-cannula systems are effective in unloading the LA, they often require more complex management and are associated with a higher risk of complications (5,7).
This approach aligns with recent literature, emphasizing the need for safer and less invasive strategies for left atrial decompression during VA-ECMO (3,8,16). By simplifying the process and minimizing the need for additional venous or arterial access, this single-cannula technique offers a practical and efficient alternative in critically ill patients. Nonetheless, the efficacy of left atrial unloading using this method remains an area for future investigation, particularly in relation to specific hemodynamic parameters and their correlation with clinical outcomes.
By simplifying LV unloading through a transseptal approach, the use of a single multistage venous drainage cannula directly addresses the challenge of LV distension on VA-ECMO by offering an efficient method for decompressing the LV. This integration with VA-ECMO reduces the risk of pulmonary edema and other complications related to inadequate LV decompression.
Strengths and limitations
One of the key strengths of this study was the demonstration of the simplicity and safety of the single-cannula transseptal technique for LV unloading in patients on VA-ECMO. By eliminating additional arterial access points, this method provides a potentially safer alternative to multidevice approaches such as ECMO and concomitant Impella support (ECPELLA), which combines VA-ECMO and Impella while ensuring effective decompression of the left ventricle (3,11). The addition of real-time TEE and fluoroscopic guidance ensures accurate cannula placement and enhances procedural safety (9,10).
However, this study has some limitations. The small sample size and retrospective study design limited the generalizability of the findings. Larger prospective studies are required to validate these outcomes and to broaden the applicability of this technique. Additionally, although no significant complications were observed during the study, long-term follow-up is necessary to evaluate the durability of the outcomes and identify any delayed complications (8,14).
Comparison with other techniques
When used in conjunction with VA-ECMO, the IABP provides additional support for LV unloading (17) but allows only modest reductions in LVEDP and pulmonary capillary wedge pressure (PCWP). Although it has a favorable safety profile, its efficacy in severe cardiac failure is limited, often necessitating the use of alternative or adjunctive unloading methods (5,7).
The Impella device, a microaxial pump, offers effective LV unloading by directly pumping blood from the LV, resulting in a significant reduction in LVEDP and pulmonary congestion. However, Impella use is associated with higher risks, such as hemolysis and vascular complications, as well as increased costs owing to its complexity (18). Managing Impella in conjunction with VA-ECMO can further complicate patient management, resulting in a higher incidence of bleeding complications and technical challenges (18-20).
Surgical techniques, such as direct LV or LA venting via thoracotomy, offer even more substantial unloading but are accompanied by greater risks of bleeding, infection, and prolonged recovery. These approaches are typically reserved for patients undergoing cardiac surgery or for those who do not respond to percutaneous unloading methods. Minimally invasive surgical techniques, such as mini-thoracotomy, offer an alternative, but still carry risks of bleeding and infection (7).
The choice between single- and dual-cannula techniques for LV unloading during VA-ECMO involves balancing procedural simplicity, safety, and the ability to achieve precise hemodynamic control. The single-cannula transseptal approach employed in this study relies on the insertion of a multiperforated venous cannula placed transseptally to simultaneously decompress the left and the right atria. This technique capitalizes on the natural pressure gradient between the left and right atria, with the left atrial pressure typically being higher, thereby promoting effective LV unloading without complex adjustments. The procedural simplicity of this method reduces the risk of complications, such as atrial wall suction or cannula displacement, which are common concerns with dual-cannula setups.
In contrast, several previous studies (3,21-23) have introduced a two-cannula technique for LV unloading by employing a second venous drainage cannula for left atrial decompression. In a large series of 62 patients, Kim et al. demonstrated that this approach improved the ECMO weaning (61.3% vs. 38.7%) and cardiac transplantation (29.0% vs. 11.3%) rates (3). Despite its demonstrated efficacy in reducing pulmonary congestion and enhancing patient outcomes, the dual-cannula approach has added procedural complexity and risks such as complications related to additional venous femoral punctures.
As highlighted by Kim et al. (3), the single-cannula transseptal approach also demonstrated comparable efficacy in reducing left atrial pressure and pulmonary congestion, while minimizing procedural risks. Their findings support the feasibility of the single-cannula technique as a streamlined alternative to LV unloading in critically ill patients requiring VA-ECMO. Although the dual-cannula approach allows for more granular control of atrial decompression (24), the simplicity and safety of the single-cannula strategy makes it a viable and practical option in scenarios where rapid deployment and minimal invasiveness are critical.
By contrast, the single multiperforated venous cannula (Medtronic 25 F Bio-Medicus NextGen cannula) used in our study combined venous drainage and LA decompression, thereby eliminating the need for separate venous access. This simplification reduces procedural complexity, while offering comparable benefits for LV unloading and hemodynamic improvement. The single-cannula approach may also lower the risk of multiple cannulations, making it a more suitable option in critically ill patients. Furthermore, it is likely to be more cost-effective owing to its fewer components and reduced procedure times, thereby enhancing its practicality in clinical settings.
Implications and future directions
The transseptal approach using a single venous multistage drainage cannula (Medtronic 25 F Bio-Medicus NextGen cannula) offers a promising alternative for LV unloading, particularly in patients at high risk of complications related to arterial access. Future studies should focus on validating these findings in larger patient populations while exploring long-term outcomes including myocardial recovery and post-ECMO survival rates. Comparative studies between this approach and other unloading strategies, such as Impella and IABP, would help clarify the advantages and potential limitations of this technique and further refine its role in clinical practice.
Conclusions
Our findings support the use of transseptal LA decompression with a single multiperforated venous cannula as a safe and effective method of LV unloading. This simplified approach may serve as a viable alternative to the dual venous cannula strategy, reduce complexity, and enhance safety using TEE guidance in a hybrid operating room, suggesting that this method may offer a viable alternative to more invasive strategies such as ECPELLA or surgical LV venting (25). By eliminating the necessity for additional arterial or venous access, the single-cannula approach minimizes the risk of vascular complications without compromising the left atrial drainage. Larger multicenter studies are necessary to confirm these findings and assess the long-term benefits of this approach in a broader patient population.
Acknowledgments
We acknowledge the multidisciplinary Heart Team at Rouen University Hospital for their contribution to the management of these patients.
Footnote
Reporting Checklist: The authors completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1993/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1993/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1993/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees, and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patients for publication of this surgical technique and accompanying images and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Russo JJ, Aleksova N, Pitcher I, et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J Am Coll Cardiol 2019;73:654-62. [Crossref] [PubMed]
- Xie A, Forrest P, Loforte A. Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation. Ann Cardiothorac Surg 2019;8:9-18. [Crossref] [PubMed]
- Kim AR, Park H, Lee SE, et al. Outcomes of left ventricular unloading with a transseptal cannula during extracorporeal membrane oxygenation in adults. Artif Organs 2021;45:390-8. [Crossref] [PubMed]
- Kawashima D, Gojo S, Nishimura T, et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J 2011;57:169-76. [Crossref] [PubMed]
- Meani P, Gelsomino S, Natour E, et al. Modalities and Effects of Left Ventricle Unloading on Extracorporeal Life support: a Review of the Current Literature. Eur J Heart Fail 2017;19:84-91. [Crossref] [PubMed]
- Truby LK, Takeda K, Mauro C, et al. Incidence and Implications of Left Ventricular Distention During Venoarterial Extracorporeal Membrane Oxygenation Support. ASAIO J 2017;63:257-65. [Crossref] [PubMed]
- Desai SR, Hwang NC. Strategies for Left Ventricular Decompression During Venoarterial Extracorporeal Membrane Oxygenation - A Narrative Review. J Cardiothorac Vasc Anesth 2020;34:208-18. [Crossref] [PubMed]
- Orozco-Hernandez EJ, Ahmed MI, Von Meering G, et al. Femoral venoarterial extracorporeal membrane oxygenation using a novel biatrial cannula for venous drainage and left ventricular venting. J Card Surg 2020;35:3631-3. [Crossref] [PubMed]
- Addis DR, Wang B, Prejean SP, et al. Utility of three-dimensional transesophageal echocardiography to guide transseptal positioning of a single multistage venous cannula to provide both venous drainage and indirect left ventricular venting in veno-arterial extracorporeal membrane oxygenation. Echocardiography 2020;37:1860-3. [Crossref] [PubMed]
- Meers JB, Nanda NC, Watts TE 3rd, et al. Utility of transesophageal echocardiography to assess real time left atrial pressure changes and dynamic mitral regurgitation following placement of transseptal multistage venous cannula for systemic venous drainage and indirect left ventricular venting in venoarterial extracorporeal membrane oxygenation. Echocardiography 2021;38:493-9. [Crossref] [PubMed]
- Schrage B, Ibrahim K, Loehn T, et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019;139:1249-58. [Crossref] [PubMed]
- Lin YN, Chen YH, Wang HJ, et al. Atrial Septostomy for Left Atrial Decompression During Extracorporeal Membrane Oxygenation by Inoue Balloon Catheter. Circ J 2017;81:1419-23. [Crossref] [PubMed]
- Baldetti L, Gramegna M, Beneduce A, et al. Strategies of left ventricular unloading during VA-ECMO support: a network meta-analysis. Int J Cardiol 2020;312:16-21. [Crossref] [PubMed]
- Donker DW, Brodie D, Henriques JPS, et al. Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion 2019;34:98-105. [Crossref] [PubMed]
- Russo G, Taramasso M, Maisano F. Transseptal puncture: procedural guidance, challenging situations and management of complications. EuroIntervention 2021;17:720-7. [Crossref] [PubMed]
- Singh-Kucukarslan G, Raad M, Al-Darzi W, et al. Hemodynamic Effects of Left-Atrial Venous Arterial Extra-Corporeal Membrane Oxygenation (LAVA-ECMO). ASAIO J 2022;68:e148-51. [Crossref] [PubMed]
- Nuding S, Werdan K. IABP plus ECMO-Is one and one more than two? J Thorac Dis 2017;9:961-4. [Crossref] [PubMed]
- Nersesian G, Tschöpe C, Spillmann F, et al. Prediction of survival of patients in cardiogenic shock treated by surgically implanted Impella 5+ short-term left ventricular assist device. Interact Cardiovasc Thorac Surg 2020;31:475-82. [Crossref] [PubMed]
- David CH, Quessard A, Mastroianni C, et al. Mechanical circulatory support with the Impella 5.0 and the Impella Left Direct pumps for postcardiotomy cardiogenic shock at La Pitié-Salpêtrière Hospital. Eur J Cardiothorac Surg 2020;57:183-8. [Crossref] [PubMed]
- Shibasaki I, Masawa T, Abe S, et al. Benefit of veno-arterial extracorporeal membrane oxygenation combined with Impella (ECpella) therapy in acute coronary syndrome with cardiogenic shock. J Cardiol 2022;80:116-24. [Crossref] [PubMed]
- Dulnuan K, Guglin M, Zwischenberger J, et al. Left atrial veno-arterial extracorporeal membrane oxygenation: percutaneous bi-atrial drainage to avoid pulmonary edema in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2018;71:A1358. [Crossref]
- Eng M, Al-Darzi W, Basir M, et al. Left atrial venous arterial extracorporeal membrane oxygenation for biventricular failure in cardiogenic shock. Eur Heart J 2021;42:1061. [Crossref]
- Rai B, Rosse CJ, Gorder KL, et al. Left Atrial Veno-Arterial Extracorporeal Membrane Oxygenation (LAVA-ECMO) is a Feasible Option for Patients in Cardiogenic Shock for Whom Impella Offloading is Contraindicated. J Heart Lung Transplant 2021;40:S522. [Crossref]
- Villablanca PA, Fadel RA, Giustino G, et al. Hemodynamic Effects and Clinical Outcomes of Left Atrial Veno-Arterial Extracorporeal Membrane Oxygenation (LAVA-ECMO) in Cardiogenic Shock. Am J Cardiol 2025;236:79-85. [Crossref] [PubMed]
- Ughetto A, Aouinti S, Molinari N, et al. Early left ventricular unloading via active transseptal left atrial venting in case of cardiogenic shock under veno-arterial extracorporeal membrane oxygenation: A meta-analysis. Eur J Heart Fail 2024;26:701-3. [Crossref] [PubMed]


