
North American expert consensus on the clinical role of ex vivo lung perfusion (EVLP) with acellular perfusate
Highlight box
Key recommendations
• Lungs that are acceptable after ex vivo lung perfusion (EVLP) are equivalent to standard criteria lungs and can be used in high-risk recipients.
• Lungs donated for transplant that have unclear or marginal quality should be placed on EVLP for evaluation.
• Placing lungs on EVLP: core parameters for this decision include compliance and deflation, the ratio of PaO2 to fraction of inspired oxygen (P/F ratio), peak inspiratory pressure (PIP), edema on imaging, and bronchoscopy, with additional parameters considered as appropriate when lung quality is in doubt.
• Transplanting EVLP lungs: core parameters for this decision include deflation and compliance, radiography, delta PO2 at the completion of EVLP, overall movement, STEEN Solution™ loss, bronchoscopy, peak airway pressure, and palpation, with additional parameters considered as needed.
• Lungs that are acceptable on all relevant parameters are appropriate for transplant; lungs that are borderline may be appropriate based on clinical judgement.
What was recommended and what is new?
• There are currently no evidence-based guidelines to support decision-making about use of EVLP in lung transplantation. Protocols and decision-making criteria vary across centers.
• This expert consensus outlines frameworks and parameters to support decision-making in the use of EVLP.
What is the implication, and what should change now?
• Use of EVLP can increase the availability of acceptable donor lungs and should be expanded. Research to define evidence-based criteria for decision-making in EVLP is needed.
Introduction
Lung transplantation is potentially lifesaving for patients with end-stage pulmonary disease. However, transplantation is limited by the marked shortage of acceptable donor lungs (1). Only 15% to 30% of donated lungs are accepted for transplantation (2-7). The lack of sufficient donor lungs leads to 16.1 deaths per 100 patient-years in patients waitlisted for lung transplant (1).
Ex vivo lung perfusion (EVLP) can potentially prolong the lives of some patients with end-stage lung disease by mitigating the shortage of donor lungs. In EVLP, explanted lungs are perfused and ventilated by an EVLP device. Two types of perfusate are used: acellular perfusates such as STEEN Solution™, and blood-based perfusates. This article focuses on EVLP with acellular perfusate, which was first used successfully clinically in 2001, and used for a transplant of an extended criteria lung in 2005 (8,9). EVLP allows assessment and potential utilization of lungs that were not accepted initially as well as those that are not assessable with standard methods due to logistical or other issues. EVLP can also address logistical constraints by extending the donor lung(s) total ischemic time, even beyond the extension that may result from the advent of 10 ℃ storage techniques for donor lungs (10,11). Multiple studies have found that outcomes with EVLP are similar to those with standard criteria non-EVLP lungs (12-20).
The use of EVLP is typically reported to increase the number of lung transplants that can be performed by approximately 20% (21). This varies across transplant centers, with one highly experienced center reporting a 70% increase (22). As a result, EVLP has become an important tool in some transplant centers, although wider use is feasible.
EVLP procedures require several key decisions. First the surgeon must determine whether the donor lung(s) should be placed on EVLP, taken straight to transplant, or rejected. If the lung(s) are placed on EVLP, the surgeon will then need to determine when or whether the lung(s) are suitable for transplant. Currently, published clinical evidence to support these decisions for EVLP with acellular perfusate is limited. The available evidence is primarily from small, uncontrolled, single-center studies. Interpreting the results of these studies is challenging because of variations between centers in critical details of the EVLP procedures. As a result, no evidence-based guidelines address these decisions, and clinicians base their decisions primarily on clinical judgment and personal and institutional experience.
In this situation, expert consensus recommendations may be helpful to clinicians involved in lung transplantation. This study is intended to help optimize the use of EVLP with acellular perfusate by convening a panel of expert physicians experienced with lung transplantation in North America. The panel developed expert consensus recommendations for placing donor lungs on EVLP, assessment of lungs on EVLP, and subsequent consideration for transplant. The study may also assist the field by highlighting open questions and potential targets for research. We note that high-quality procurement and recovery procedures are essential for successful lung transplantation with or without EVLP but are beyond the scope of this manuscript.
Methods
The expert consensus recommendations presented here were developed by a panel of 18 lung transplant surgeons and pulmonologists who practice in North America and have experience in EVLP. The consensus study was conceived by the lead author (M.B.) and senior author (K.R.M.) in cooperation with the sponsor (Lung Bioengineering Corporation, Inc., Silver Spring, MD, USA). Potential panel members were nominated by the lead and senior authors based on experience with EVLP using acellular perfusate and expertise in the field of lung transplantation, focusing on institutions using the two primary acellular perfusate systems used in North America (XVIVO XPS™ and Traferox) and those using centralized EVLP services. A total of 19 physicians were nominated. All 19 were invited to participate and completed Survey 1. One panelist, who is experienced with EVLP but not with acellular perfusate, withdrew after the first survey. The remaining 18 panelists (14 transplant surgeons and four pulmonologists) completed all three surveys. The panel was moderated by the lead and senior authors with support from an experienced medical writer and OPEN Health Group.
Table 1 summarizes the experience of the 18 expert panelists. Figure S1 provides additional detail.
Table 1
| Type of lung transplantation experience | Length of experience | Lungs transplanted | |||
|---|---|---|---|---|---|
| Classification | Panelists, n [%] | Classification | Panelists, n [%] | ||
| Any type of lung transplantation | <10 years | 2 [11] | <300 | 4 [22] | |
| 10–19 years | 12 [67] | 300–699 | 9 [50] | ||
| ≥20 years | 4 [22] | ≥700 | 5 [28] | ||
| Lung transplantation using EVLP | <5 years | 2 [11] | <20 | 4 [22] | |
| 5–10 years | 11 [61] | 20–99 | 10 [56] | ||
| ≥11 years | 5 [28] | ≥100 | 4 [22] | ||
| Lung transplantation using EVLP with acellular perfusate | <5 years | 3 [17] | <20 | 6 [33] | |
| 5–10 years | 11 [61] | 20–99 | 8 [44] | ||
| ≥11 years | 4 [22] | ≥100 | 4 [22] | ||
EVLP, ex vivo lung perfusion.
Authorship criteria for all panelists were (I) completion of all three surveys; (II) contribution to development of the article [attendance at ≥1 manuscript review meeting(s) and review and comment on ≥1 draft(s) of the article]; and (III) review and approval of the final manuscript. No panelists were compensated for participation in the study. All manuscript review meetings were conducted virtually.
The consensus recommendations are based primarily on a modified Delphi process that used three surveys. The surveys were developed by the lead and senior authors with medical writing support from OPEN Health Group and implemented in the SurveyMonkey® platform. Panelists were provided with a link to each survey by email and asked to respond independently and anonymously. The surveys covered three broad areas: general questions on EVLP, decision criteria for placing donor lungs on EVLP, and decision criteria for transplanting EVLP lungs. Figure S2 presents a graphical overview of the study process.
Survey 1 consisted primarily of open-ended, qualitative questions to elicit panelists’ opinions and practices on indications for EVLP, evaluation of lungs on EVLP, and other relevant topics. The questionnaire was written by OPEN Health Group based on interviews with the lead and senior authors (M.B. and K.R.M.) and a literature search, followed by review and revision by the lead and senior authors. After panelists completed the survey, OPEN Health Group consolidated and anonymized the results and developed an executive summary. The results and summary were reviewed by the lead and senior authors.
Survey 2 was based on the results of Survey 1. It used several question types, including Likert-scale questions in which panelists rated their agreement with statements about EVLP on an 11-point scale from −5 (strongly disagree) to +5 (strongly agree) or could choose “Not applicable”; “Select all that apply”, multiple-choice, and yes/no questions to address items from Survey 1 that could not be fully assessed with Likert-scale questions; and open-response questions at the end of each topic area and the end of the survey. Each “Select all that apply” question included an “other” choice that requested an open response. The lead and senior authors were provided with the consolidated, anonymized results of Survey 2 and an executive summary.
Consensus was predefined for Likert-scale questions, as a mean score ≥2.5 or ≤−2.5 with a standard deviation (SD) that does not cross zero [i.e., SD less than the absolute value of the mean (Figure S3)]. Near agreement was defined post hoc as a Likert-scale score ≥2.25 or ≤−2.25. Except where noted, scores are reported as mean ± SD.
Survey 3 was identical to Survey 2, except that a few additional questions were added to clarify points deemed insufficiently covered in Survey 2. In addition, panelists were provided with their answers from Survey 2 along with the mean and SD of the answers from the entire panel. This information may promote agreement by allowing individual panelists to compare their scores against the group’s. The lead and senior authors were provided with the consolidated and anonymized results and an executive summary.
Survey 3 included a total of 174 questions, including 159 Likert-scale, 1 ranking, 11 multiple-choice, and 3 open-response. Of the 159 Likert-scale questions, panelists reached agreement on 54 (34%), near agreement on 7 (4%), no agreement on 97 (61%), and agreement against on 1 (1%). Table available at https://cdn.amegroups.cn/static/public/jtd-2024-2069-1.docx presents a detailed listing of the questions and results from Survey 3.
On reviewing the Survey 3 results, the lead and senior authors identified several open questions including the roles of EVLP in donor lungs with pulmonary embolism or infarction and in normothermic regional perfusion (NRP) donation after cardiocirculatory death (DCD) and made a post hoc decision to invite panelists to a virtual follow-up meeting to discuss them. The virtual meeting was attended by 14 panelists and led to the development and distribution of a short, emailed questionnaire. Participation in the meeting and short questionnaire were not required for authorship.
A total of 14 panelists attended the virtual follow-up meeting to discuss open questions, and 10 answered the short questionnaire distributed after the meeting.
General statements on EVLP
Statement 1: the primary goals for EVLP are (I) expanding the number of donor lungs available for transplant; (II) use as a research tool; and (III) improving the function and quality of otherwise marginal lungs.
The panel was surveyed on the importance of four possible goals for EVLP: expanding the number of donor lungs available, use in research, improving lung function and quality, and improving logistical flexibility, and reached consensus that expanding donor lung availability is the most important goal.
The panel considered the role of EVLP for management of lungs that meet standard criteria. No panelists use EVLP to improve the quality of standard criteria lungs, although some use EVLP in this setting to address logistical or other considerations.
Statement 2: lungs that are acceptable after EVLP are equivalent to standard criteria lungs and can be used in high-risk recipients.
The panel reached near consensus that decisions on intraoperative and postoperative management should be based on patient and lung status rather than EVLP history. However, some panelists manage recipients of EVLP lungs differently than recipients of non-EVLP lungs.
The panel did not explore these differences in depth. However, many panelists take care to control lung reperfusion and excessively rapid increases in blood flow to the lung, particularly for lungs with less than ideal parameters. Strategies vary across panelists but may include allowing the lung to rest on veno-arterial extracorporeal membrane oxygenation longer before decannulation in the operating room, use of epoprostenol or inhaled nitric oxide, and/or higher levels of positive end-expiratory pressure.
Decision criteria for placing donor lungs on EVLP
Recommendation 1: lungs with unclear or marginal quality should be placed on EVLP for evaluation. Lungs that meet standard criteria should go straight to transplant unless logistical issues require EVLP. Lungs that are unacceptable should be rejected outright.
Each of these possibilities requires nuanced decision-making and application of clinical judgement. Situations where one donor lung does not meet standard criteria for transplant and the original intended recipient requires a double lung transplant are more complicated, and panelists did not make a consensus recommendation: most panelists would put both lungs on EVLP, particularly if the injured lung is potentially recoverable. In other situations, these panelists would find a single lung recipient. A minority of panelists would reject the non-standard criteria lung and find a recipient for the good lung.
Recommendation 2: EVLP is recommended for evaluation of donor lungs that have been recovered by third-party organizations which provided only incomplete or concerning information.
EVLP is a valuable tool for in-depth assessment of lungs with uncertain or marginal quality.
Recommendation 3: the following parameters should always be considered in decisions on placing lungs on EVLP: compliance and deflation, the ratio of PaO2 to fraction of inspired oxygen (P/F ratio), peak inspiratory pressure (PIP), edema on imaging, and bronchoscopy. Additional parameters should be considered as appropriate when lung quality is unclear, including but not limited to: edema on palpation, the type of lung injury (e.g., contusion vs. aspiration), other radiographic findings, the possibility of aspiration, donor type [donation after brain death (DBD) vs. DCD], the possibility of pulmonary emboli, recipient characteristics, and cold ischemia time 1 (CIT1; time from cross-clamping to reperfusion by EVLP) should be considered in appropriate situations.
Figure 1 details the results of the survey questions leading to these recommendations. Panelists did not reach consensus on specific threshold criteria for any of these parameters.
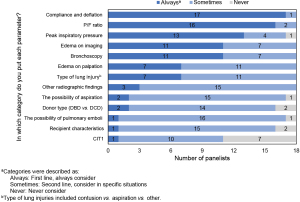
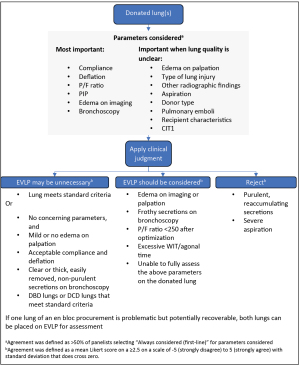
Decisions regarding the disposition of donor lungs require nuanced consideration of numerous parameters. In this study, the expert panel generally follows current practice in expanding the “standard criteria” for taking lungs straight to transplant and suggesting additional assessments that can supplement decision making: edema on palpation, compliance and deflation, and the acceptability of thick, easily removed, non-purulent secretions (23). In lungs where a decision to accept or reject outright is unclear, EVLP can evaluate and possibly condition the lung and potentially increase the number of available donor lungs.
The panelists identified several specific situations in which EVLP can be useful for evaluation of “non-standard” lungs: moderate or severe edema, high PIP, frothy secretions, low P/F ratio, and excessive warm ischemia time (WIT)/agonal time. Figure 2 outlines the panel’s recommended general approach to the decision on whether to put lungs on EVLP.
Decision criteria for transplanting EVLP lungs
Recommendation 4: EVLP lungs that are acceptable on all relevant parameters are appropriate for transplant. EVLP lungs that are borderline on some parameters may be appropriate for transplant depending on clinical judgment and the specific clinical scenario. Bilateral EVLP lungs where parameters are borderline for one lung may be appropriate for transplant depending on clinical judgment and the specific scenario.
Recommendation 5: the following parameters should always be considered in decisions about transplantation of lungs that have been placed on EVLP: deflation and compliance, radiography, delta PO2 at the completion of EVLP, overall movement, STEEN Solution™ loss, bronchoscopy, peak airway pressure, and palpation. Other parameters including pulmonary vascular resistance (PVR), weight gain, left atrial (LA) PO2 at the completion of EVLP, glucose and lactate, and estimated cold ischemia time 2 (CIT2; time from removal from EVLP to reperfusion) should be considered in appropriate situations. Panelists did not reach consensus on specific threshold criteria for any of these parameters.
The above recommendations are based on a series of survey questions in which panelists rated each factor as always considered (first-line), sometimes considered (second-line), or never considered. Figure 3 shows the results.
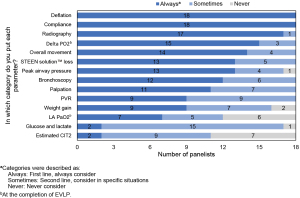
EVLP allows a detailed assessment of the donor lung. However, there is currently no clear guidance on appropriate parameters or criteria for decisions about suitability for transplant. This study suggests a set of parameters and criteria (Table 2). Lungs that are stable or improving on EVLP and acceptable on all of these criteria are suitable for transplant. Lungs that are deteriorating on EVLP and/or do not meet all of these criteria should be evaluated based on clinical judgment and the specific situation.
Table 2
| Parameters | Criteria |
|---|---|
| Deflation | Complete; normal or near-normal rate |
| Compliance | <15% deterioration |
| Edema | No new or significant edema by imaging or palpation |
| Blood gases | Delta PO2 >350 mmHg or PaO2 >350 mmHg |
| • Near consensus for delta PaO2 >300 mmHg | |
| Overall movement | Acceptable |
| • Poor movement in a few segments should be considered acceptable | |
| STEEN Solution™ loss | Acceptable |
| • >200 mL in the first hour is acceptable if it slows or stops by the next assessment | |
| Bronchoscopy | Clear |
| • Near consensus that nonrecurrent nonpurulent secretions are acceptable | |
| Peak airway pressure | <15% increase |
| PVR | <15% increase |
| Weight gain | <15–20% increase |
| Causes for concern | • Severely abnormal or rapidly changing glucose or lactate |
| • Diffuse abnormalities on imaging |
EVLP, ex vivo lung perfusion; PVR, pulmonary vascular resistance.
The considerations for EVLP and criteria for transplant acceptability suggested here overlap with criteria suggested in other publications (2,21,24). Several scoring systems for lungs on EVLP have been evaluated. The COMPLETE score is the sum of scores from 10 parameters, each scored from 0 (best) to 3 (worst). In a study of 73 EVLP cases, the COMPLETE score with a cut-off of 12 had 100% sensitivity and specificity for transplant suitability (25). All of the parameters used in the COMPLETE score except ultrasound are also recommended here. An inflammation score based on interleukin (IL)-6 and IL-8 levels in perfusate predicted the suitability of lungs for transplant and recipient outcomes (26). The EXPIRE regression model predicts lung suitability with good discriminative power after 4 hours of EVLP (27). Machine-learning algorithms have been applied to optimize EVLP assessment. In a blinded, retrospective study, the InsighTx AUROC model gave excellent results in evaluating the suitability of donor lungs for transplant (28).
EVLP research needs and open questions
EVLP has been in use for decades but is still undergoing rapid development and faces a significant lack of robust, multicenter controlled trials. As a result, decisions about EVLP must rely on a limited base of clinical evidence. The panel identified several important gaps in the EVLP evidence base. Key needs include specific evidence-based criteria for placing lungs on EVLP, improved strategies and tools for lung selection and assessment, the potential role of biomarkers and other nonclinical parameters in decision making, and enhanced techniques and perfusion solutions to improve lung quality and function (e.g., removal of emboli, treatment of infections, and immunomodulation) and prolong the useful duration of EVLP.
The panel discussed but could not reach consensus on several important aspects of EVLP practice. Areas of disagreement like this are to be expected for cutting-edge questions in rapidly evolving like EVLP and indicate open questions for which additional research is needed.
Use of EVLP in donor lungs with pulmonary embolism or infarction
For lungs with pulmonary embolism/infarction, EVLP may be used for additional monitoring and/or as a platform for interventions to clear emboli. Use of EVLP in this situation was discussed in the virtual panel meeting and addressed in the follow-up survey (Table S1). Of the 10 panelists who responded to the survey, six place lungs on EVLP if blood clots appear in the pre-EVLP retrograde flush or if there are other signs of pulmonary embolism, and five of these sometimes use EVLP to clear emboli (e.g., with thrombolytics or vascular angioextraction). The potential role of EVLP for clearing emboli warrants additional research.
Large pulmonary infarction, in contrast, should trigger rejection of the lung: eight of nine panelists who responded to this survey reject lungs with large areas of infarction.
Use of EVLP in NRP donation after circulatory death
The use of EVLP with NRP DCD lungs was also discussed in the virtual panel meeting and with a two-question survey during the meeting (Table S2). Panelists did not reach consensus on the role of EVLP for NRP DCD lungs and concluded that the field’s experience of EVLP with NRP is insufficient for confident recommendations, particularly given the rapid evolution of NRP technique.
Cold ischemia time (CIT)
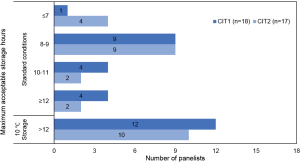
Panelists considered CIT (time from cross-clamping to placement of the lung in recipient’s chest cavity in lungs taken straight to transplant), CIT1 and CIT2, and their role in decision making. In responses to Survey 3, panelists agreed that lungs with CIT <7 hours can be taken straight to transplant but did not reach agreement on longer CITs, or on the roles of CIT1 or CIT2 in decision making. These topics were explored in the virtual panel meeting after Survey 3 and a follow-up survey. Panelists noted that these decisions are complex, multifactorial, and nuanced. Most panelists feel that lungs can probably be stored for longer than is usually done in current practice, particularly if EVLP is used in conjunction with 10 ℃ storage (10,29).
When surveyed about the maximum acceptable CIT and CIT2, a majority of the panel indicated that storage for up to 8–9 hours is acceptable in standard storage conditions, and that ≥12 hours is acceptable at 10 ℃ storage (Figure 4 and Tables S3,S4). Note that panelists did not define CIT2 uniformly. Panelists agreed that CIT2 starts with removal of the lung from EVLP but use three distinct endpoints: placement of the lung in the recipient’s chest cavity, placement of the first stitch of the anastomosis, and reperfusion of the first lung. A uniform consensus definition of CIT2 will be important for comparisons across multiple trials. Most panelists identified lung quality, procurement quality, and storage technique as key factors in decisions about CIT and CIT2.
Prone positioning for EVLP lungs
Data from animal models and anecdotal clinical reports suggest that prone positioning of lungs during EVLP may be beneficial. However, the available clinical data is insufficient for a firm recommendation and prone positioning is not used routinely. Some programs with relevant expertise use prone positioning in specific circumstances, such as EVLP lungs with lower lobe edema.
Conclusions
Lung transplantation techniques are evolving rapidly. Notably, the role of EVLP continues to evolve as rapid improvements in the field of lung transplantation are considered, such as 10 ℃ storage, NRP procurement, improved procurement and transplant technique, and improved EVLP systems and perfusates (29,30). These evolutionary changes are unlikely to change the core decision-making considerations highlighted by these consensus recommendations.
Lungs that are acceptable after EVLP are equivalent to lungs that meet standard criteria initially. EVLP should be used to evaluate donor lungs with unclear or marginal quality. In this role, EVLP can increase the number of donor lungs available for transplantation. Decisions about placing lungs on EVLP require application of clinical judgement and consideration of multiple parameters including assessments of lung function, structure, and history. Additional research is needed to better define specific decision criteria and address the role of EVLP in the evolving field of lung transplantation.
Acknowledgments
We would like to thank OPEN Health group for editorial and logistical support, and Edward K. Baldwin, PhD, for medical writing support.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2069/prf
Funding: The study was sponsored and supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2069/coif). B.A.W. serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2023 to July 2025. All authors report that the study was sponsored and supported by Lung Bioengineering, Inc. M.B. has received grants from the National Institutes of Health, Congressionally Directed Medical Research Programs, and Cystic Fibrosis Foundation; is a board member for Xylyx Bio, Inc. and the Chief Medical Officer (unpaid, no intellectual property, patents, nor stock) for ART, Inc.; and holds several issued or pending patents related to EVLP and transplantation. C.A.B. has received consulting fees for serving on medical advisory boards for Abbot and Abiomed. A.W.B. has received grants from and is a part-time employee of the Cystic Fibrosis Foundation, received consulting fee from Lung Bioengineering, Inc., and has served as a volunteer on the OPTN Lung Transplantation Committee Region 2 and the OPTN Lung Exception Review Board. M.M.B. has participated on an advisory board for Lung Bioengineering, Inc. and Transmedics, Inc., and has held leadership roles as the UNOS Lung Committee Chair and on the UNOS Multiorgan Committee and UNOS Placement Efficiency Taskforce. M.C. has acted as a consultant for Lung Bioengineering, Inc., Incyte, and Avivo, and is the Chief Scientific Officer for and a shareholder in Traferox Technologies Inc. S.K. is the Chief Medical Officer for Traferox Technologies Inc., a consultant for United Therapeutics Corp., Lung Bioengineering, Inc., CareDx, Abbott, and CSL Behring, and holds pending or issued patents on EVLP technologies and lung diagnostic biomarkers. Z.N.K. is the Chief Executive Officer of ProCure On-Demand, Inc. He holds stock in ProCure On-Demand, Inc. and notes that Lung Bioengineering, Inc. is a minority investor. J.M.M. has consulted for Lung Bioengineering, Inc. (payments made to the Mayo Clinic, his employer). T.K.W.’s institution has received research grants and royalties or licenses from United Therapeutics Corp. He has received consulting fees from Lung Bioengineering, Inc., and has several patents related to EVLP. He is a founder of Traferox Technologies, Inc., and holds stock and stock options in the company. B.A.W. has received grants from the National Institutes of Health and participated on the Clinical Events Committee for Transmedics Inc. K.R.M. has received royalties related to intellectual property from XVIVO; received lecture honoraria from Lung Bioengineering Inc. and XVIVO; and received support for attending meetings from XVIVO; holds pending or issued patents related to lung evaluation and EVLP; has a volunteer leadership role on the UNOS/OPTN Board of Directors; and has stock or stock options in TransMedics, Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2020 Annual Data Report: Lung. Am J Transplant 2022;22:438-518. [Crossref] [PubMed]
- Watanabe T, Cypel M, Keshavjee S. Ex vivo lung perfusion. J Thorac Dis 2021;13:6602-17. [Crossref] [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [Crossref] [PubMed]
- Tuttle-Newhall JE, Krishnan SM, Levy MF, et al. Organ donation and utilization in the United States: 1998-2007. Am J Transplant 2009;9:879-93. [Crossref] [PubMed]
- Yeung JC, Cypel M, Waddell TK, et al. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin 2009;19:261-74. [Crossref] [PubMed]
- Snell GI, Griffiths A, Levvey BJ, et al. Availability of lungs for transplantation: exploring the real potential of the donor pool. J Heart Lung Transplant 2008;27:662-7. [Crossref] [PubMed]
- Sung RS, Galloway J, Tuttle-Newhall JE, et al. Organ donation and utilization in the United States, 1997-2006. Am J Transplant 2008;8:922-34. [Crossref] [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg 2007;83:2191-4. [Crossref] [PubMed]
- Ali A, Wang A, Ribeiro RVP, et al. Static lung storage at 10°C maintains mitochondrial health and preserves donor organ function. Sci Transl Med 2021;13:eabf7601. [Crossref] [PubMed]
- Ali A, Hoetzenecker K, Luis Campo-Cañaveral de la Cruz J, et al. Extension of Cold Static Donor Lung Preservation at 10°C. NEJM Evid 2023;2:EVIDoa2300008.
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Jawitz OK, Raman V, Becerra D, et al. Lung Transplantation After Ex Vivo Lung Perfusion Early Outcomes From a US National Registry. Ann Surg 2022;275:1006-12. [Crossref] [PubMed]
- Luo Q, Zhu L, Wang Y, et al. The Conversional Efficacy of Ex Vivo Lung Perfusion and Clinical Outcomes in Patients Undergoing Transplantation of Donor Lungs by Ex Vivo Lung Perfusion: A Meta-Analysis. Ann Transplant 2019;24:647-60. [Crossref] [PubMed]
- Divithotawela C, Cypel M, Martinu T, et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg 2019;154:1143-50. [Crossref] [PubMed]
- Tian D, Wang Y, Shiiya H, et al. Outcomes of marginal donors for lung transplantation after ex vivo lung perfusion: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2020;159:720-730.e6. [Crossref] [PubMed]
- Ghaidan H, Fakhro M, Andreasson J, et al. Ten year follow-up of lung transplantations using initially rejected donor lungs after reconditioning using ex vivo lung perfusion. J Cardiothorac Surg 2019;14:125. [Crossref] [PubMed]
- Zhang ZL, van Suylen V, van Zanden JE, et al. First experience with ex vivo lung perfusion for initially discarded donor lungs in the Netherlands: a single-centre study. Eur J Cardiothorac Surg 2019;55:920-6. [Crossref] [PubMed]
- Nilsson T, Wallinder A, Henriksen I, et al. Lung transplantation after ex vivo lung perfusion in two Scandinavian centres. Eur J Cardiothorac Surg 2019;55:766-72. [Crossref] [PubMed]
- Mallea JM, Hartwig MG, Keller CA, et al. Remote ex vivo lung perfusion at a centralized evaluation facility. J Heart Lung Transplant 2022;41:1700-11. [Crossref] [PubMed]
- Possoz J, Neyrinck A, Van Raemdonck D. Ex vivo lung perfusion prior to transplantation: an overview of current clinical practice worldwide. J Thorac Dis 2019;11:1635-50. [Crossref] [PubMed]
- Cypel M, Yeung JC, Donahoe L, et al. Normothermic ex vivo lung perfusion: Does the indication impact organ utilization and patient outcomes after transplantation? J Thorac Cardiovasc Surg 2020;159:346-355.e1. [Crossref] [PubMed]
- Okahara S, Levvey B, McDonald M, et al. Common Criteria for Ex Vivo Lung Perfusion Have No Significant Impact on Posttransplant Outcomes. Ann Thorac Surg 2021;111:1156-63. [Crossref] [PubMed]
- Niikawa H, Okamoto T, Ayyat KS, et al. Significant parameters in the evaluation of donor lungs in single-lung cellular ex vivo lung perfusion. Interact Cardiovasc Thorac Surg 2019;28:767-74. [Crossref] [PubMed]
- Ayyat KS, Okamoto T, Sakanoue I, et al. The complete score for comprehensive evaluation of donor lungs in ex-vivo lung perfusion: an approach for optimizing the outcomes of transplantation. J Heart Lung Transplant 2022;41:S192. [Crossref]
- Sage AT, Richard-Greenblatt M, Zhong K, et al. Prediction of donor related lung injury in clinical lung transplantation using a validated ex vivo lung perfusion inflammation score. J Heart Lung Transplant 2021;40:687-95. [Crossref] [PubMed]
- Di Nardo M, Del Sorbo L, Sage A, et al. Predicting donor lung acceptance for transplant during ex vivo lung perfusion: The EX vivo lung PerfusIon pREdiction (EXPIRE). Am J Transplant 2021;21:3704-13. [Crossref] [PubMed]
- Sage AT, Donahoe LL, Shamandy AA, et al. A machine-learning approach to human ex vivo lung perfusion predicts transplantation outcomes and promotes organ utilization. Nat Commun 2023;14:4810. [Crossref] [PubMed]
- Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med 2017;5:119-24. [Crossref] [PubMed]
- Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J Heart Lung Transplant 2017;36:744-53. [Crossref] [PubMed]


