
Photodynamic therapy as a promising treatment for long-segment and whole-circumferential early esophageal cancer
Highlight box
Key findings
• Photodynamic therapy (PDT) is effective and safe for long-segment and whole-circumferential early esophageal squamous cell carcinoma (ESCC).
• A longer tumor length (>10 cm) is identified as a risk factor of PDT failure for whole-circumferential early ESCC.
• For patients with whole-circumferential early ESCC, the subsequent esophageal stricture after PDT treatment seemed to be easier to resolve than that after endoscopic submucosal dissection (ESD) treatment, yielding a shorter dysphagia duration.
What is known and what is new?
• ESD is widely accepted as a standard treatment for early esophageal cancer given the low risk of lymph node metastasis. However, circumferential resections are typically concerned or even avoided because of the high risk of persistent stricture formation, especially when the lesion’s length was over 5 cm.
• PDT may be considered as a promising therapeutic modality for long-segment and whole-circumferential early ESCC, which presents advantages in the occurrence and control of the subsequent esophageal stricture over ESD.
What is the implication, and what should change now?
• The findings in our study may bring more attention to clinical application of PDT and more exploration of the related clinical efficacy for early esophageal cancer. If carefully excluding metastasis, PDT could be used as an alternative to surgery in patients with whole-circumferential early esophageal cancer, especially for the elderly or those with many comorbidities. Prospective and large-scale multicenter studies are expected in the future.
Introduction
Esophageal cancer is one of the most common malignant neoplasms in the world, ranking seventh in terms of incidence and sixth in mortality overall (1). Earlier detection of esophageal cancer and better utilization of therapeutic modalities are important to improve patients’ long-term survival and quality of life (QOL) (2). As gastroenterologists, we focus on the endoscopic management of early esophageal cancer, defined as cancer limited to the mucosa and submucosa without lymph node metastasis. The endoscopic therapies for early esophageal cancer include endoscopic resection (ER), photodynamic therapy (PDT) and cryotherapy thermal ablation (3).
Nowadays, endoscopic submucosal dissection (ESD) is widely accepted as a standard treatment for early esophageal cancer (4-7). However, lesions involving the whole circumference of the esophagus, especially those with a major axis length over 5 cm, come into a dilemma due to intractable esophageal stricture (7). Esophagectomy has been recommended as the mainstay of curative treatment for such extensive early esophageal cancer or salvage treatment for post-ESD refractory esophageal stricture (4,8,9). PDT is the combination treatment involving a photosensitizing agent (photosensitizer) with an affinity for tumors and a photodynamic reaction subsequently triggered by laser light in the presence of oxygen (10). PDT is currently more frequently used as a palliative treatment in advanced esophageal cancers and a salvage treatment for local failure following chemoradiotherapy (CRT) or radiotherapy (RT) (10-13). Local complete response (CR) has been reported even in considerable cases with invasion of muscularis propria (3,10). PDT is also indicated for curative therapy for dysplastic Barrett’s esophagus and early esophageal cancer (10,11). However, with the rapid development of ESD, PDT has somewhat lost favor in choice for early esophageal cancer over the last few years. PDT treatment for whole-circumferential early esophageal cancer has been rarely reported. We therefore intended to investigate the efficacy and complications of PDT for the whole-circumferential early esophageal squamous cell carcinoma (ESCC). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1967/rc).
Methods
Patients
We reviewed patients with the whole-circumferential early ESCC who received PDT between January 2020 and July 2022 at the First Affiliated Hospital with Nanjing Medical University. All the participants should be prospectively followed up for more than one year. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital with Nanjing Medical University (No. 2021-SR-274) and individual consent for this retrospective analysis was waived.
Patients in our study should be preoperatively diagnosed as early ESCC limited to the mucosa or upper third of submucosa (≤200 µm) without lymph node metastases. Before PDT, computed tomography (CT) was performed to exclude the presence of nodal or distant metastasis. The tumor depth was evaluated by two independent experienced gastroenterologists according to the combination of the macroscopic morphology under white-light imaging (WLI), the morphological characteristics of intrapapillary capillary loops (IPCL) showed by magnifying endoscopy with narrow band imaging (NBI), the “tatami” sign after Lugol staining or the tumor echo under endoscopic ultrasonography (EUS) (7,14-16). WLI was initially used to evaluate findings such as surface irregularity and thickness. Lesions with an almost flat or slightly uneven surface (≤1 mm) are considered to be no more than upper third of submucosa (SM1). Such flat type could be classified according to the Paris Workshop guidelines (types 0–IIa, IIb, IIc). When magnifying endoscopy is used, the invasion depth is diagnosed based on the Japan Esophageal Society (JES) classification (16). If the entire lesion is composed of type B1 vessels or avascular area (AVA)-small, the lesion is diagnosed as epithelial (EP)/lamina propria mucosa (LPM); if type B2 vessels or AVA-middle are observed, the lesion is diagnosed as muscularis mucosa (MM)/SM1; and if type B3 vessels or AVA-large are observed, the lesion is diagnosed as cancer invading >200 µm into the submucosa. In addition, “tatami” sign could be used to differentiate LPM and MM. In our study, the preoperative diagnosis of the invasion depth was divided into two categories: T1a-EP/LPM and T1a-MM/T1b-SM1. Take Figure 1 as an example; the patient was diagnosed as T1a-EP/LPM. Moreover, tumor length was recorded simultaneously. All the patients should meet the above diagnostic criteria of early esophageal cancer and have no intention for initial ESD, surgery, chemotherapy, or radiation. We then excluded patients that didn’t regularly receive gastroscopy and chest CT after PDT or lost follow-up.

As a reference or control group, we simultaneously included the contemporaneous patients with early ESCC using whole-circumferential ESD in the hope of exploring the curative resection rate and the concomitant complications, especially the post-ESD esophageal stricture.
PDT procedure
Hematoporphyrin derivative (commodity name: Hipofin) was administrated at a dose of 2 or 2.5 mg/kg. Then PDT was performed 40–48 hours later. The boundaries of the targeted lesion were firstly demarcated by argon plasma coagulation (APC) (ERBE, Tuebingen, Germany), and consecutively the laser light at 635 nm wavelength (GYS-PST-S2000, Guoyi Huake, Tianjin, China) was delivered circumferentially via a cylindrical optical fiber through gastroscope (GIF-H260J, GIF-H260Z, GIF-Q260J, GIF-Q260Z, GIF-HQ290, GIF-H290Z, Olympus Co. Ltd., Tokyo, Japan). Multiple treatment fields were overlapped to cover the lesion according to its size and then got irradiated in succession. During the PDT procedure, the recorded fluence rate of the laser light was set to 200–500 mW/cm at a light dose of 150–300 J/cm of tumor area. On the following day, the gastroscopic observation was performed to partially remove the necrosis and further check for residual lesions (Figure 1). If a residual lesion was found, additional laser irradiation was warranted. All the participants were advised to receive a follow-up gastroscopy examination for response evaluation after 2–3 months. If not cured, they would be recommended for a second course of PDT treatment (Figure S1).
ESD procedure
Firstly, lesions should be evaluated by magnifying endoscopy with NBI, and then the boundaries of the lesion should be demarcated by Lugol staining. Multiple spots were marked at 3–5 mm away from the margin of the lesion by using DualKnife (KD-650Q; Olympus, Japan) to ensure a clear safety margin. Then, normal saline plus indigo-carmine with 0.0025% epinephrine was injected into the submucosa using a 23-gauge disposable injector to raise the lesion. A circumferential incision and subsequent submucosal dissection were conducted with the DualKnife (KD-650Q; Olympus, Japan). Finally, hemostatic forceps (FD-410LR; Olympus, Japan) was used in a soft coagulation mode (60–80 W output) to control bleeding.
Evaluation
The criteria for CR were as follows: (I) disappearance of PDT-induced ulcer and scar formation was confirmed; (II) no residual tumor was observed; (III) disappearance of cancer cells was assessed histologically by biopsy and chromoendoscopy (Lugol staining or NBI) could be an auxiliary method to evaluate CR (7,16).
Dysphagia manifested as the disability to swallow caused by esophageal stricture. The Stooler’s dysphagia score was used to classify the severity, as follows: 0, able to eat a normal diet; 1, able to eat some solid food; 2, able to eat semi-solid food only; 3, able to swallow liquids only; and 4, unable to swallow anything. We regarded the remission criteria of esophageal stricture as the dysphagia score descending to grade 0 to 1 with gastroscopy could pass through for reexamination, and without relapse of dysphagia being observed for another 8 weeks.
Follow-up
The initial follow-up endoscopy was recommended for efficacy evaluation after 2–3 months. If cured, patients would be suggested to regularly receive endoscopic observations and chest CT every 6 months in the first year and then yearly afterward. Targeted biopsy should be obtained in situ or with the guidance of chromoendoscopy (Lugol staining or NBI). Lugol-voiding lesions (LVLs) would be paid more attention in the follow-up period. The following endoscopic interval may be changed according to both the degree of LVLs and the targeted pathology. In cases of stenosis, Lugol staining may be avoided immediately after dilation, but NBI would be used as an auxiliary method to evaluate metachronous recurrence instead. Lugol staining would be recommended 2–3 months later. Through electronic medical records and multiple telephone follow-up survey, the short-term and long-term adverse events caused by endoscopic treatment were documented. Furthermore, we paid special attention to the occurrence of esophageal stricture. The patients would be informed of possible complication and resolvent of dysphagia or esophageal stricture before endoscopic treatment. And they were further reminded to pay attention to diet and seek medical help immediately in case of dysphagia serious. If the patient visits or contacts us, a priority to admission for required treatment would be provided. Finally, therapeutic modality of esophageal stricture, the time when dysphagia initially appeared and finally got alleviated, the first time and frequency of endoscopic dilation, and the change of dysphagia scores would be recorded.
Statistical analysis
The statistical analysis was conducted using SPSS version 25.0 for Windows. Continuous data were presented as the mean ± standard deviation (SD) and analyzed by the unpaired t-test or rank sum test. Categorical variables were expressed as proportion (%) and analyzed by the Fisher’s exact test. The difference of post-endoscopic esophageal stricture duration between the PDT and ESD groups was compared by Kaplan-Meier method. A two-tailed P<0.05 was considered to be statistically significant.
Results
Patient’s characteristics and response rate
We successfully included 12 patients with the whole-circumferential early ESCC who received PDT. The clinicopathological features of these patients are summarized in Table 1. The pre-PDT biopsy histopathology of all the patients were squamous epithelium high-grade intraepithelial neoplasia or carcinosis in situ. There are 6 men and 6 women, with a median age of 72 years old [interquartile range (IQR), 57–84 years] at the beginning of the follow-up period. Among all, 11 of 12 patients were accompanied with past medical history, including hypertension, diabetes mellitus, atrial fibrillation, liver cirrhosis, postgastrectomy, rheumatoid arthritis, splenic artery aneurysm and chronic cardiac or renal insufficiency. In addition, two patients had a prior history of ESD. The median tumor length was 9.5 cm (IQR, 3–18 cm). All cases except one had long-segment lesions (>5 cm). All the long-segment lesions more than 10 cm and half of the T1a-MM/T1b-SM1 lesions were treated with two courses. Finally, all the patients received more than once reexamination of chest CT and gastroscopy in the follow-up period. A proportion of 83.33% of patients were evaluated as CR after a median follow-up of 28 months (IQR, 17–43 months). The response rate of each group was presented in Table 1. No significant differences were observed in gender, age, past medical history, macroscopic type, or preoperative evaluated invasion depth (P value is shown in Table 1). But there was a statistically significant difference in tumor length. A longer tumor length (>10 cm) was a risk factor of PDT failure (P=0.045).
Table 1
| Characteristics | N | CR | P value |
|---|---|---|---|
| Sex | |||
| Male | 6 | 5 (83.33) | >0.99 |
| Female | 6 | 5 (83.33) | |
| Age (years) | |||
| 45–64 | 1 | 1 (100) | >0.99 |
| ≥65 | 11 | 9 (81.82) | |
| Past medical history | |||
| Yes | 11 | 9 (81.82) | >0.99 |
| None | 1 | 1 (100) | |
| Tumor length (cm) | |||
| ≤10 | 9 | 9 (100) | 0.045* |
| >10 | 3 | 1 (33.33) | |
| Macroscopic type | |||
| Flat | 10 | 9 (90) | 0.32 |
| Flat + elevated | 2 | 1 (50) | |
| Pre-PDT diagnosis of depth | |||
| T1a-EP/LPM | 8 | 7 (87.5) | >0.99 |
| T1a-MM/T1b-SM1 | 4 | 3 (75) | |
| PDT courses per patient | |||
| 1 | 8 | 8 (100) | 0.09 |
| 2 | 4 | 2 (50) | |
Data are presented as n (%). *, the result was statistically significant. CR, complete regression; EP, epithelial; ESCC, esophageal squamous cell carcinoma; LPM, lamina propria mucosa; MM, muscularis mucosa; PDT, photodynamic therapy; T1a, intramucosal carcinoma; T1b-SM1, invasive carcinoma into the submucosa no more than 200 μm.
Due to the same assessment criteria of indication for both endoscopic modalities, we therefore simultaneously investigated preoperative diagnostic accuracy based on the postoperative pathology of the contemporaneous patients with whole-circumferential early ESCC that received ESD for reference. The information was presented in Table 2. Though en bloc resection rate was 100%, the lesions were deeper than expected in 13 of 60 (21.67%) cases.
Table 2
| Outcome of ESD | Data (N=60) |
|---|---|
| Non-curative resection | 13 (21.67) |
| Non-curative factor | |
| Deep submucosal infiltration | 9 |
| Lymphovascular invasion | 1 |
| Both of above | 3 |
| Curative resection | 47 (78.33) |
| Stenosis intervention | |
| None | 8 |
| Oral glucocorticoids | 14 |
| Drug injection | 8 |
| Automucosal transplantation | 3 |
| Incision of annular muscle | 10 |
| Unknown | 4 |
Data are presented as n, or n (%). ESCC, esophageal squamous cell carcinoma; ESD, endoscopic submucosal dissection.
Adverse events and management of post-endoscopic esophageal stricture
After injection of photosensitizer, patients were educated to avoid intense sunlight for at least 1 month. No skin phototoxicity, bleeding, or perforation happened. Chest pain appeared to be the most common immediate adverse reaction to PDT, followed by hydrothorax and fever. Esophageal stricture remained to be the most common adverse event in the curative cases after PDT. Finally, there were no new lymph node metastases during follow-up, but one patient relapsed in situ and received PDT again.
All the patients that received PDT didn’t have any prophylactic measures for stricture formation. In contrast, prophylactic measure was taken in most curative patients after ESD treatment (Table 2). Except for the patients undergoing further endoscopic intervention immediately after ESD, one patient that received further radiotherapy and another who did not conduct regular follow-up gastroscope, 20 curative patients after ESD treatment were included into the following comparison with those receiving PDT for adverse events. Twenty curative patients after ESD treatment were included in the following comparison with those receiving PDT for adverse events. The result is shown in Table 3.
Table 3
| Patient factor | PDT (N=12) | ESD (N=20‡) | P value |
|---|---|---|---|
| Characteristics of patients | |||
| Sex (male) | 6 (50) | 18 (90) | 0.03* |
| Age (years) | 72.42±7.10 | 66.90±8.06 | 0.06 |
| Past medical history | 11 (91.67) | 6 (30) | 0.001* |
| Procedure time (min) | 59.75±37.55 | 149.25±69.48 | 0.001* |
| Adverse events | |||
| Esophageal pain | 75 (9/12) | 35 (7/20) | 0.07 |
| Hydrothorax | 16.67 (2/12) | 0 | 0.13 |
| Fever | 8.33 (1/12) | 30 (6/20) | 0.21 |
| Bleeding | 0 | 10 (2/20) | 0.52 |
| Perforation | 0 | 0 | – |
| Stricture of curative cases | 80 (8/10) | 85 (17/20) | >0.99 |
| None prophylaxis | 80 (8/10) | 100 (7/7) | 0.26 |
| Oral glucocorticoids | – | 76.9 (10/13) | >0.99† |
| Recurrence | 10 (1/10) | 5 (1/20) | >0.99 |
Data are presented as mean ± standard deviation, n (%) or % (n/N). *, the results were statistically significant; †, the comparison is between stricture rate in PDT group and the steroid administration group of ESD treatment; ‡, N=20 from the patients without further endoscopic intervention immediately after ESD in Table 2, excluding one patient that received further radiotherapy and another who did not conduct regular follow-up gastroscope. ESD, endoscopic submucosal dissection; PDT, photodynamic therapy.
Compared with the ESD group, patients in the PDT group had more concomitant diseases (P=0.001). It took much less time to complete PDT treatment (PDT 59.75±37.55 min vs. ESD 149.25±69.48 min, P=0.001). The reaction of postoperative fever and bleeding appeared less in the PDT group (P=0.21 and 0.52, respectively), whereas chest pain and hydrothorax seemed to be more frequent (P=0.07 and 0.13, respectively). Nevertheless, there was no significant difference in the adverse events. There is a difference in the esophageal stricture prophylaxis in the PDT and ESD groups. Though without prophylactic measures, the esophageal stricture rate was 80% in the PDT group, with no significant difference compared with that in the steroid administration group of ESD treatment (P>0.99).
Regarding clinical outcomes of the post-endoscopic esophageal stricture, there is a significant difference in the onset time of obvious dysphagia and the associated therapeutic modalities (as shown in Table 4). The mean onset time of dysphagia was 7.63±4.34 and 4.29±2.49 weeks respectively in the PDT and ESD groups (P=0.02). Endoscopic dilation treatment in the follow-up period was performed on demand. Much more patients in the ESD group received esophageal stent to relief from dysphagia (P=0.042). Seven of 8 patients in the PDT group received remission of dysphagia only by endoscopic bougie or balloon dilation, and the corresponding average sessions of dilation were 4.57±2.76. A difference in the duration of post-endoscopic esophageal stricture was further discovered by using the Kaplan-Meier method between the two groups (Figure 2). The result in Table 5 shows that the estimated mean time to dysphagia remission is 12.72±3.04 and 23.69±3.24 months respectively in PDT and ESD group (P=0.08).
Table 4
| Parameters | PDT (N=8) | ESD (N=17) | P value |
|---|---|---|---|
| Tumor length (cm) | 8.25±2.71 | 7.12±2.34 | 0.29 |
| Procedure time (min) | 48.75±20.72 | 152.06±73.89 | 0.001* |
| Follow-up period (months) | 29.75±7.61 | 27.88±9.01 | 0.62 |
| Onset time to obvious dysphagia (weeks) | 7.63±4.34 | 4.29±2.49 | 0.02* |
| Treatment of esophageal stricture | 0.042* | ||
| Bougie or balloon dilation | 87.5 (7/8) | 41.18 (7/17) | |
| Esophageal stent after dilation | 12.5 (1/8) | 58.82 (10/17) |
Data are presented as mean ± standard deviation or % (n/N). *, the results were statistically significant. ESD, endoscopic submucosal dissection; PDT, photodynamic therapy.
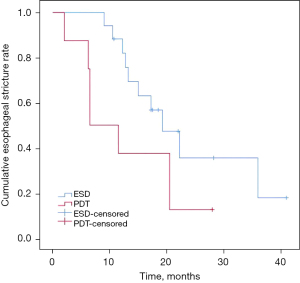
Table 5
| Therapeutics | Estimated mean value (months) | SD (months) | 95% CI | χ2 | P value |
|---|---|---|---|---|---|
| PDT | 12.72 | 3.04 | 6.77–18.67 | 3.06 | 0.08 |
| ESD | 23.69 | 3.24 | 17.33–30.05 |
P value was evaluated by log-rank test. CI, confidence interval; ESD, endoscopic submucosal dissection; PDT, photodynamic therapy; SD, standard deviation.
Discussion
ESD is now widely used as an alternative to surgery in patients with early esophageal carcinoma given the low risk of lymph node metastasis. However, circumferential resections are typically concerned or even avoided because of the high risk of persistent stricture formation, especially when the lesion’s length was over 5 cm (7,17). Furthermore, muscle layer damage is another risk factor associated with the refractory stricture after whole-circumferential esophageal ESD (17,18). No conclusive prophylactic therapy has been clear for the prevention of esophageal stricture after whole circumferential ER yet (19). During ESD procedure, the established excisional boundary is usually demarcated 3–5 mm away from the lesion, thus the actual excisional length is longer than estimated to ensure a clear safety margin. In addition, muscle layer damages may occur sometimes. The more damages to normal tissue during ESD may cause a greater dilemma for the following esophageal stricture. In addition to esophagectomy as a recommended treatment for such extensive lesion, CRT is often an alternative treatment for early ESCC depending on patients’ comorbidities, tumor localization, and extensive extension (4,20). But the potential recurrence and toxicities after RT brings concern (21). PDT is another effective endoscopic therapy indicated for early esophageal cancer and has been reported to yield good results even in cases with suspected invasion of the submucosa or muscularis propria (3). PDT is a two-stage procedure. The first stage is an administration of a light-sensitive photosensitizer, which could be taken up by malignant or dysplastic tissues with selectivity. The second stage is light irradiation to the targeted tissues, then resulting in photochemical reactions. In the presence of oxygen, the photoactivated photosensitizer leads to the formation of reactive oxygen species (ROS) that rapidly reacts with cellular components, causing oxidative damage and ultimately leading to the death of tumor cells. This procedure is often associated with secondary effects such as damage to the microvasculature and stimulation of anti-tumor immunity (22). Moreover, the whole procedure was theoretically specific to tumor tissues and reduced damage to normal tissues (Figure 1G,1H); thus, we wonder whether PDT could be an effective and safe treatment for the long-segment and whole-circumferential early esophageal cancer.
In our study, twelve patients diagnosed as whole-circumferential early ESCC received successful follow-up. For these elderly patients with diverse concomitant diseases and long-segment lesions, the CR rate was 83.3%. The CR rate was similar to that in the previous study concerning extensive early ESCC lesions judged difficult for ER (23). Though the accurate histopathologic assessment cannot be acquired after PDT, we further examined the preoperative diagnostic accuracy of whole-circumferential early ESCC in contemporaneous ESD group for reference. The result showed that 21.67% of the lesions were deeper than expected. The higher CR rate of PDT treatment indicates that PDT may be effective in early ECSS deeper than SM1 when excluding metastasis. Two of 12 patients failed after two courses of PDT treatment. Both patients had longer tumor lengths (>10 cm) and received two PDT courses. Upon further analysis, a longer tumor length (>10 cm) was identified as a risk factor of PDT failure. In addition, the patient who relapsed was the third one who had longer tumor length (>10 cm). PDT induced significant apoptosis in ESCC cells in a time- and dose-dependent manner (24). We assume longer or larger lesions may need higher doses of photosensitizer administration or higher doses of light irradiation. All that mentioned above suggests that PDT can be a curative treatment for whole-circumferential early ESCC, but we should be more cautious about PDT treatment for longer lesions (>10 cm) and pay more attention to the following endoscopy reexamination. Multiple, deeper, or even ultrasound-guided biopsies may be required for CR evaluation of longer lesions. Even if CR is not achieved, a good prognosis can be expected if suitable additional treatment is administered. Just as the previous studies (25,26), we observed that the second course of PDT was also safe and effective for early ESCC, but two patients still failed after two courses of PDT treatment. It remains a question that whether a third PDT course may be attempted into practice.
Moreover, PDT can be considered as a safe treatment option in adverse events compared with ESD treatment. The most common adverse event after PDT is esophageal stricture, which could cause dysphagia and result in decreased QOL (27). Post-ESD stricture often inevitably occur after whole-circumferential esophageal resection without preventive measures. Oral steroid administration is a strategy option that could be beneficial for the prevention and control of postoperative stricture after whole-circumferential esophageal ESD (19,28). In our study, the esophageal stricture rate in the post-PDT group was comparable to that in the ESD group with steroid administration. Furthermore, dysphagia appeared later in the PDT group and the post-PDT esophageal strictures were resolved more easily with a simpler dilation modality instead. Though without a significant difference in Kaplan-Meier analysis, the estimated dysphagia duration in the post-PDT group tended to be shorter. The above-mentioned indicated that PDT was more beneficial in the aspect of the post-endoscopic stricture formation and control. In our opinion, the potential advantage of PDT may come from the high specificity of photosensitizer and light irradiation to tumor tissues. However, chest pain and hydrothorax appeared more frequently after PDT treatment in our study. We speculate that it may result from an excessive and rapid tumor necrosis after PDT, which remains to be a question between the benefit-to-harm balance in the future.
As an observational study, the selection bias of patients’ characteristics, endoscopic modality adoption and post-operative stricture prophylaxis was obvious. Generally, patients that considered not suitable enough for ESD and prophylactic measures are more likely to receive PDT on account of physical weakness or some potential risks related to past medical history. As mentioned above, the procedure time of PDT was much shorter than ESD, which is more friendly to elderly or weak patients. In this case, PDT treatment is usually considered as a safe endoscopic modality for patients with early ESCC, especially the elderly accompanied with comorbidities. Furthermore, post-operative stricture prophylaxis was actually more taken in the ESD group, but the dysphagia occurred later and was relieved earlier and easier after PDT treatment. The above bias is more favorable for PDT treatment as a promising endoscopic modality for patients with whole-circumferential early ESCC. The second limitation in our study is that PDT cannot allow for a precise pathological assessment after the procedure, but all the participants were carefully evaluated before endoscopic treatment and prospectively followed up for more than one year. In the follow-up period they received gastroscopy and chest CT for several times, especially those requiring hospitalization for esophageal dilation. So, the CR rate is credible enough. Another limitation is that the frequency of endoscopic dilation in the two groups was not compared due to a limited number of patients. In fact, the number of whole-circumferential ESCC patients who met our research criteria was limited overall; furthermore, PDT treatment was not broadly recognized. PDT was also reported as the initial treatment for Barrett’s esophagus and early esophageal adenocarcinoma (29,30), but there are few studies on whole-circumferential lesions to date. Prospective and large-scale multicenter studies regarding short-term and long-term outcomes of PDT treatment for whole-circumferential early esophageal cancer are expected in the future.
There have been few studies comparing PDT and ESD treatment for esophageal cancer so far. One study reported that ESD and PDT as salvage treatments after definitive CRT for superficial ESCC have the similar curative effect and long-term prognosis (31). Another study proposed that PDT may be comparable to the ESD for early esophageal cancer. With the exception of esophageal stricture, PDT could reduce many complications and have longer disease-free survival in comparison with ESD (32). But the whole-circumferential lesions investigated in this study were very limited. In our study, we primarily focused on the clinical outcomes concerning ESD and PDT treatment for the whole-circumferential early ESCC. We further found that PDT may present advantages in the occurrence and control of the subsequent esophageal stricture over ESD treatment for long-segment and whole-circumferential lesions.
Conclusions
PDT may be considered as a promising therapeutic modality for long-segment and whole-circumferential early ESCC, especially for the elderly or those with many comorbidities.
Acknowledgments
The authors are very grateful to all the patients and families that agreed to participate in the study. We thank all healthcare staff working in Department of Gastroenterology and Endoscopy Center of the First Affiliated Hospital with Nanjing Medical University, Nanjing, China.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1967/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1967/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1967/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1967/coif). All authors report funding from the Open subject of Jiangsu Health Development Research Center (No. JSHD2022041). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the First Affiliated Hospital with Nanjing Medical University (No. 2021-SR-274) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen R, Liu Y, Song G, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut 2021;70:251-60. [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus 2019;16:25-43.
- Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus 2023;20:343-72.
- Mizumoto T, Hiyama T, Oka S, et al. Curative Criteria After Endoscopic Resection for Superficial Esophageal Squamous Cell Carcinomas. Dig Dis Sci 2018;63:1605-12. [Crossref] [PubMed]
- Yamauchi K, Iwamuro M, Nakagawa M, et al. Long-term outcomes of endoscopic versus surgical resection for MM-SM1 esophageal squamous cell carcinoma using propensity score analysis. Esophagus 2021;18:72-80. [Crossref] [PubMed]
- Ishihara R, Arima M, Iizuka T, et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc 2020;32:452-93. [Crossref] [PubMed]
- Min YW, Lee H, Song BG, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointest Endosc 2018;88:624-33. [Crossref] [PubMed]
- Fugazza A, Repici A. Endoscopic Management of Refractory Benign Esophageal Strictures. Dysphagia 2021;36:504-16. [Crossref] [PubMed]
- Yano T, Wang KK. Photodynamic Therapy for Gastrointestinal Cancer. Photochem Photobiol 2020;96:517-23. [Crossref] [PubMed]
- Wu H, Minamide T, Yano T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig Endosc 2019;31:508-16. [Crossref] [PubMed]
- Yano T, Kasai H, Horimatsu T, et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017;8:22135-44. [Crossref] [PubMed]
- Minamide T, Yoda Y, Hori K, et al. Advantages of salvage photodynamic therapy using talaporfin sodium for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Surg Endosc 2020;34:899-906. [Crossref] [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1-24.
- Gruner M, Denis A, Masliah C, et al. Narrow-band imaging versus Lugol chromoendoscopy for esophageal squamous cell cancer screening in normal endoscopic practice: randomized controlled trial. Endoscopy 2021;53:674-82. [Crossref] [PubMed]
- Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017;14:105-12. [Crossref] [PubMed]
- Miwata T, Oka S, Tanaka S, et al. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc 2016;30:4049-56. [Crossref] [PubMed]
- Geng ZH, Zhu Y, Li QL, et al. Muscular injury as an independent risk factor for esophageal stenosis after endoscopic submucosal dissection of esophageal squamous cell cancer. Gastrointest Endosc 2023;98:534-542.e7. [Crossref] [PubMed]
- Zou J, Chai N, Linghu E, et al. Prevention of Esophageal Stricture After Whole Circumferential Endoscopic Resection: A Review for Endoscopists. Turk J Gastroenterol 2022;33:811-21. [Crossref] [PubMed]
- Haneda R, Booka E, Ishii K, et al. Evaluation of Definitive Chemoradiotherapy Versus Radical Esophagectomy in Clinical T1bN0M0 Esophageal Squamous Cell Carcinoma. World J Surg 2021;45:1835-44. [Crossref] [PubMed]
- Kawamoto T, Shikama N, Mine S, et al. Clinical outcomes of definitive radiotherapy for patients with cT1aN0M0 esophageal cancer unsuitable for endoscopic resection and surgery. J Gastrointest Oncol 2022;13:454-61. [Crossref] [PubMed]
- Donohoe C, Senge MO, Arnaut LG, et al. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer 2019;1872:188308. [Crossref] [PubMed]
- Tanaka T, Matono S, Nagano T, et al. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc 2011;73:1-6. [Crossref] [PubMed]
- Gao S, Zhang M, Zhu X, et al. Apoptotic effects of Photofrin-Diomed 630-PDT on SHEEC human esophageal squamous cancer cells. Int J Clin Exp Med 2015;8:15098-107. [PubMed]
- Yamashita H, Kadota T, Minamide T, et al. Efficacy and safety of second photodynamic therapy for local failure after salvage photodynamic therapy for esophageal cancer. Dig Endosc 2022;34:488-96. [Crossref] [PubMed]
- Lecleire S, Di Fiore F, Antonietti M, et al. Nonoperable patients with superficial esophageal cancer treated by photodynamic therapy after chemoradiotherapy have more severe complications than patients treated in primary intent. Am J Gastroenterol 2008;103:2215-9. [Crossref] [PubMed]
- Ishihara R. Prevention of esophageal stricture after endoscopic resection. Dig Endosc 2019;31:134-45. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011;11:46. [Crossref] [PubMed]
- Wolfsen HC, Hemminger LL, Wallace MB, et al. Clinical experience of patients undergoing photodynamic therapy for Barrett's dysplasia or cancer. Aliment Pharmacol Ther 2004;20:1125-31. [Crossref] [PubMed]
- Gray J, Fullarton G. The current role of photodynamic therapy in oesophageal dysplasia and cancer. Photodiagnosis Photodyn Ther 2007;4:151-9. [Crossref] [PubMed]
- Shiota J, Yamaguchi N, Isomoto H, et al. Long‑term prognosis and comprehensive endoscopic treatment strategy for esophageal cancer, including salvage endoscopic treatment after chemoradiation therapy. Exp Ther Med 2023;25:121. [Crossref] [PubMed]
- Hua X, Li Y, Ma H, et al. Photodynamic therapy versus endoscopic submucosal dissection for management of patients with early esophageal neoplasia: a retrospective study. J Thorac Dis 2017;9:5046-51. [Crossref] [PubMed]


