
miR-124/PTBP1/PKM axis modulates pulmonary artery endothelial metabolism in a pulmonary thromboembolism rat model
Highlight box
Key findings
• Rats with pulmonary thromboembolism (PTE) exhibited disrupted miR-124/PTBP1/PKM signaling, leading to elevated lactate levels and pulmonary arterial intimal remodeling.
• miR-124 overexpression improved endothelial metabolism by reducing PTBP1/PKM2 activity and restoring pyruvate utilization.
What is known and what is new?
• Metabolic dysfunction in pulmonary artery endothelial cells (PAECs) contributes to chronic thromboembolic pulmonary hypertension (CTEPH). PTBP1 and PKM2 are implicated in pathological glycolysis.
• This study identifies miR-124 as a novel regulator of the PTBP1/PKM axis in PTE, linking metabolic reprogramming to vascular remodeling, and demonstrates miR-124’s therapeutic potential.
What is the implication, and what should change now?
• Targeting the miR-124/PTBP1/PKM axis may prevent metabolic dysfunction and CTEPH progression.
• Future studies should validate findings in human PAECs and assess long-term therapeutic effects of miR-124 activation.
Introduction
Pulmonary embolism (PE) encompasses a group of conditions or clinical syndromes resulting from the blockage of the pulmonary artery or its branches by various emboli, with pulmonary thromboembolism (PTE) being one of the most prevalent forms of PE (1). Endothelial cell metabolism is a key factor in determining endothelial cell phenotype and function. Metabolic dysfunction in pulmonary artery endothelial cells (PAECs) plays a crucial role in the pathological and physiological progression from PE to chronic thromboembolic pulmonary hypertension (CTEPH).
Pyruvate occupies a central role in the cellular metabolic network (2). Pyruvic acid is synthesized through the action of pyruvate kinase (PK), which exists in most cell types as two isozymes, PKM1 and PKM2. The low-activity PKM2 dimer promotes glycolysis by facilitating the conversion of pyruvate to lactate, while PKM1 and the highly active PKM2 tetramers promote the tricarboxylic acid cycle (TCA cycle) process (3). The expression status of PKM subtypes is regulated by three heteronuclear ribonucleoproteins (hnRNPs), including PTBP1 (which referred to as hnRNPI), hnRNPA1, and hnRNPA2 (4).
These hnRNPs are downstream targets of micro-ribonucleic acids (miRNAs), such as microRNA-124 (miR-124), with PTBP1 being a specific miR-124 target (5,6). Currently, the roles and interactions of miR-124, PTBP1, PKM1, and PKM2 in the progression of PE to CTEPH remain unclear. Consequently, this study hypothesizes that the miR-124/PTBP1 axis may influence endothelial cell metabolic programming by modulating the PKM2/PKM1 balance during the progression from PE to CTEPH, thereby contributing to pulmonary arterial intimal remodeling. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1806/rc).
Methods
Model establishment
Animals
Eighteen healthy male Sprague Dawley (SD) rats, aged 2 months and weighing between 250 to 300 g, were provided by the Experimental Animal Center of Fujian Medical University (Fuzhou, China). The rats were maintained in indoor environments with temperatures ranging from 20 to 24 ℃ and a relative humidity of 65–70%. All animals have unrestricted activities and can enjoy food and water at any time. A protocol was prepared before the study without registration. All experimental procedures and animal care protocol were approved by the Animal Ethics Committee of Fujian Medical University Institutional Animal Care and Use Committee (IACUC FJMU 2024-Y-0941) and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Bethesda, MD, USA).
Groups
The 18 rats were randomly assigned to one of three groups: control (n=6), PTE (n=6), and PTE + miR-124 activator (n=6). Inject the obtained autologous thrombus into the left external jugular vein of the PTE group rats, with additional injections on days 3 and 5 following the initial procedure. A mixture of 32±6 blood clots suspended in 1 mL of physiological saline was administered via the left external jugular vein at a rate of 0.2 mL/min. The blood clots were cylindrical in shape. The control group underwent the same procedure but received only physiological saline without clots (7). The procedure for the PE + miR-124 activator group was similar, with 50 optical density (OD) of the miR-124 activator (purchased from Gemma Gene, Wuhan, China) injected through the external jugular vein along with the clot and saline mixture during the initial injection and on day 3. Penicillin (10,000 U/kg/day) was administered for three days post-surgery for infection prevention. Endogenous fibrinolysis system was inhibited by daily intra-abdominal injections of tranexamic acid (TXA) (200 mg/kg) until the rats were euthanized. Observe all animals for 2 weeks and collect specimens.
Sample collection
After anesthetization, 2 mL of arterial blood was gathered from the abdominal aorta, and ethylene diamine tetraacetic acid (100 g/L) was contribute as an anticoagulant. After centrifugation at 3,000 rpm and 4 ℃, the blood was stored at −80 ℃ for 15 minutes. Lung tissues were dissected for further analysis. After thoracotomy, the lungs were carefully extracted, and the pulmonary artery was gently flushed with sterile saline before fixation. Separate half of the lung tissue and fix it in formalin (10%), then embed it in paraffin and perform hematoxylin and eosin (HE) staining and immunohistochemistry. The remaining lung tissue was used to isolate pulmonary arteries, which were stored at −80 ℃ for subsequent real-time quantitative polymerase chain reaction (RT-qPCR) and western blot analysis.
Image analysis
Lung tissues were isolated and selectively fixed in 10% formaldehyde for 24 hours. The tissues were then embedded in paraffin, sliced, stained with HE, dehydrated, and subsequently photographed. The images were captured and processed using a computer-based image acquisition system, comprising a light microscope and a digital medical image analysis system.
Plasma lactate concentration
Lactic acid levels in rat plasma were measured using an L-Lactic Acid (LA) Colorimetric Assay Kit (Elabscience Biotechnology Co., Ltd., Houston, TX, USA), following the manufacturer’s protocol.
Immunohistochemistry
Pulmonary artery sections underwent deparaffinization and rehydration using an alcohol gradient. Following blocking and antigen retrieval, the sections were cultivated with rabbit anti-rat PTBP1, PKM1, PKM2, MPC1, and MPC2 polyclonal antibodies (1:50, 1:100, and 1:200, respectively; Abcam, Cambridge, UK). Subsequently, the sections were processed using the biotin-streptavidin horseradish peroxidase (HRP) detection system (ZSGB-BIO, Shanghai, China) in accordance with the manufacturer’s instructions. The first and second antibodies were omitted to serve as negative controls for each slide group.
RT-qPCR
RT-qPCR was employed to detect the expression of miR-124, PTBP1, PKM1, PKM2, MPC1, and MPC2 in pulmonary arterial intimal tissue samples, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as the internal control. The experimental procedure followed standard RT-qPCR protocols.
Western blot analysis
The protein expression levels of PTBP1, PKM1, PKM2, MPC1, and MPC2 were evaluated by using western blot analysis. Protein concentrations were measured using the Lowry protein assay and proteins were isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After blocking with BSA, the membranes were incubated overnight at 4 ℃ with anti-rat PTBP1, PKM1, PKM2, MPC1, and MPC2 (1:500, 1:500, and 1:1,000, respectively; Abcam). The membranes were then incubated with HRP-conjugated rabbit secondary antibodies for two hours. Use enhanced chemiluminescence reagent method to detect target antigens and the proteins were analyzed by using the Lab-Work image analysis software (Gene Company Limited, Hong Kong, China).
Statistical analysis
Statistical analysis was performed using SPSS 24.0 (IBM, Armonk, NY, USA). Numerical parameters following a normal Gaussian distribution were expressed as mean ± standard deviation (SD). Comparisons within groups were made using variance analysis, and the Pearson correlation coefficient was used to assess correlations between variables. P values <0.05 were considered statistically significant.
Results
Pathological changes in different subgroups of rat models
In the control group, no thrombi were identified in the pulmonary arterial lumen, and the arterial intima was thin and without significant pathological changes (see Figure 1A). In contrast, the PTE group exhibited residual undissolved thrombi within the pulmonary arteries, accompanied by thrombus organization, recanalization, pulmonary arterial lumen narrowing, and disrupted cell arrangement (see Figure 1B). Pathological changes in the pulmonary arteries of rats in the PTE + miR-124 activator group were markedly reduced compared to the PTE group (see Figure 1C).

PTBP1, PKM1, PKM2, and MPC1 and MPC2 expression in pulmonary arteries
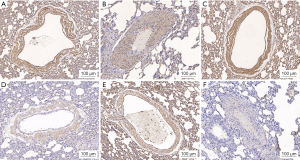
Immunohistochemistry was performed to assess the expression of PTBP1, PKM1, PKM2, MPC1, and MPC2 in pulmonary arterial intima. Expression levels of PKM1, MPC1, and MPC2 were significantly reduced in the PTE group compared to the control and PTE + miR-124 activator groups. In contrast, PTBP1 and PKM2 expression was higher in the PTE group than in the control and PTE + miR-124 activator groups (see Figures 2,3).

Plasma lactate concentration
Plasma lactate levels were the lowest in the control group and showed a significant increase in the PTE group. Lactate levels in the PTE + miR-124 activator group were significantly lower compared to those in the PTE group (P<0.05) (see Figure 4).

PTBP1, PKM1, PKM2, MPC1, and MPC2 expression in pulmonary arterial intima
Compared to the control group, the PTE group demonstrated decreased expression of PKM1, MPC1, and MPC2, alongside increased expression of PTBP1 and PKM2. The PTE + miR-124 activator group showed elevated levels of PKM1, MPC1, and MPC2, with reduced PTBP1 and PKM2 expression relative to the PTE group (see Figure 5).

Expression levels of miR-124, PTBP1, PKM1, PKM2, MPC1, and MPC2 mRNA in rat pulmonary arterial intima
The PTE group exhibited lower miR-124, PKM1, MPC1, and MPC2 and higher PTBP1 and PKM2 mRNA expression compared to the control group. The PTE + miR-124 activator group showed higher miR-124, PKM1, MPC1, and MPC2 and decreased PTBP1 and PKM2 mRNA levels compared to the PTE group (see Figure 6).

Correlation analysis
Spearman’s rank correlation analysis revealed positive correlations between lactate, PKM2, and PTBP1 levels, while negative correlations were observed between PKM1 and PTBP1 levels in the PTE group (see Table 1).
Table 1
| Variables | r | P |
|---|---|---|
| Lactate and PTBP1 | 0.855 | 0.03 |
| PKM1 and PTBP1 | −0.869 | 0.03 |
| PKM2 and PTBP1 | 0.947 | 0.004 |
Discussion
After the occurrence of acute pulmonary thromboembolism (APE), unresolved thrombi evolve into chronic obstructions within the pulmonary arteries, leading to endothelial cell damage, increased pulmonary vascular resistance, and elevated pressure, eventually progressing to CTEPH (1,8,9). CTEPH is a form of group 4 pulmonary arterial hypertension (PAH) with a high mortality rate (10). Multiple reasons leading to endothelial dysfunction in the CTEPH process, including chronic thrombosis, inflammation response, infection, metabolic abnormalities, and impaired angiogenesis function (11).
According to current literature, no animal model fully replicates the mechanism of CTEPH. Our research team developed an innovative CTEPH rat model by injecting autologous thrombi into the pulmonary arteries, simulating the pathological and physiological processes of CTEPH secondary to APE in clinical practice (7,12). In this study, residual undissolved thrombi were observed in the pulmonary arteries of rats with PTE, some showing signs of thrombus organization and recanalization. PAECs were closely associated with the thrombi, aligning with our previous findings that demonstrated pathological changes in the pulmonary arterial intima following PTE (7,12).
The metabolic plasticity of cells, defined as their dynamic ability to adapt to environmental stress, is a crucial feature of cellular physiology. Thus, the various cells that constitute the pulmonary vascular wall may regulate metabolic and functional responses to environmental stress and injury through distinct mechanisms (13). For instance, pulmonary artery adventitial fibroblasts from cows with severe idiopathic pulmonary hypertension display unique metabolic phenotypes (14). Therefore, different cells within the pulmonary vascular wall may utilize different mechanisms to regulate metabolic changes during thrombosis (9,15). However, the specific mechanisms by which these metabolic changes contribute to pulmonary arterial intimal remodeling remain unknown.
Lactic acid, a byproduct of glucose metabolism, serves as a marker of energy metabolism dysregulation. Elevated lactic acid levels are indicative of metabolic dysfunction, while reduced levels signal improvements in tissue oxygenation and energy metabolism (16). Pyruvate is the direct and sole precursor of lactic acid, the final product of glycolysis, as well as the starting point of the TCA cycle, making it a key intermediate in energy metabolism within in the body. As the central component of the entire metabolic network, pyruvate’s production and distribution play a crucial role in determining the metabolic state of cells (2). Pyruvate is primarily transported into the mitochondria via pyruvate carriers (MPCs) which are composed of two highly conserved subunits, MPC1 and MPC2 and utilized in the TCA cycle. Decreased expression and activity of MPCs is a fundamental characteristic of cancer cell metabolism. In this study, reduced MPC1 and MPC2 expression and elevated lactate levels were observed in the pulmonary arterial intima of the PTE group, suggesting metabolic alterations following PE that lead to impaired glucose metabolism and lactate accumulation. This indicates that miR-124 activators can affect the increased expression of lactate, MPC1, and MPC2, which may be involved in energy metabolism.
PK is an enzyme that can generate pyruvate and adenosine triphosphate (ATP) and is a key determinant of cellular phenotypic metabolic changes (3). It exists in most cells as two isozymes, PKM1 and PKM2, which differ functionally. PKM1 exists in highly active tetramers, while PKM2 can exist in both low activity dimers and highly active tetramers (17). The low-activity PKM2 dimer promotes glycolysis by facilitating the conversion of pyruvate to lactate, whereas PKM1 and high-activity PKM2 tetramers promote the TCA cycle process (3). In PAH, PKM1 expression significantly decreases in fibroblasts, whereas PKM2 expression remains unchanged (14). Elevated PKM2 expression or an increased PKM2/PKM1 ratio is essential for tumor cell growth and the continuous reprogramming of glycolysis and mitochondrial metabolism (3).
The expression of PKM subtypes is regulated by three hnRNPs, namely, PTBP1 (hnRNPI), hnRNPA1, and hnRNPA2. PTBP1 is involved in cell proliferation, division, embryonic development, and neuronal differentiation. In cancer cells, PTBP1 inhibits PKM1 while promoting PKM2 expression, facilitating the switch between subtypes, which in turn increases aerobic glycolysis and cell proliferation (18,19). In this study, PTE was associated with significantly decreased PKM1 expression and increased PKM2 and PTBP1 expression. PTBP1 expression was negatively correlated with PKM1 and positively correlated with PKM2 in the pulmonary arterial intima tissue. These findings indicate that energy metabolism during the progression of PE to CTEPH may mirror the metabolic alterations seen in tumors, characterized by increased cellular glycolysis.
MicroRNAs (miRNAs) are a class of short-stranded, non-coding RNAs that control gene expression levels by combine to the 3'-untranslated regions (3'-UTRs) of mRNAs, leading to either the inhibition of translation or the promotion of mRNA degradation (20). miRNAs play a role in the pathobiology of PAH by influencing cell proliferation, apoptosis, and mitochondrial metabolism (21). PTBP1 is identified as a target of miR-124, which regulates PAH fibroblast proliferation through its direct target with PTBP1 and inhibits the growth of colorectal cancer cells through the PTBP1/PKM1/PKM2 feedback cascade (6,14). The miR-124/PTBP1/PKM signaling axis regulates the metabolism and functional status of PAECs and blood growth endothelial cells (BOECs) in human idiopathic and familial pulmonary hypertension. Overexpression of miR-124 or silencing of PTPB1 has been shown to restore normal proliferation and glycolysis in familial PAH BOECs, correcting glycolytic gene and lactate dysregulation and partially restoring mitochondrial respiration (21). Furthermore, miR-124 overexpression can prevent and reverse hypoxia-induced PAH (5,22). The study observed that miR-124 expression was significantly reduced in the PTE group compared to the control group. After treatment with the miR-124 activator, miR-124, PKM1, MPC1, and MPC2 expression increased, whereas PTBP1 and PKM2 expression decreased. These results strongly suggest that miR-124 and PTBP1 play important roles in the abnormal energy metabolism of the pulmonary arterial intimal cells in PE, potentially by modulating pyruvate metabolism.
There are some limitations in this study: it examined miR-124 only at the tissue level rather than in PAECs, and it assessed only the short-term effects of PE on energy metabolism, with long-term effects requiring further investigation. We have not demonstrated that in this model the animals develop pulmonary hypertension. The lack of functional measurements of cellular metabolism and a clinically relevant effect (e.g., change in pulmonary artery pressure, right ventricular size/function, etc.) make it difficult to interpret how the observations here are relevant to human disease and warrant further study. The timing of administration of miR-124 concurrently with the thrombi makes this a study of primary prevention of CTEPH and not treatment. In the later research, further design will be carried out on the issue of administration time between treatment measures and interventions. Future research should focus on elucidating the specific mechanism of the miR-124/PTBP1/PKM axis in PAECs throughout the progressive stages of PE.
Conclusions
In conclusion, rats with PTE exhibited elevated lactate levels, decreased miR-124 expression in the pulmonary arterial intima, and abnormal energy metabolism, similar to observations in cancer cells. These findings indicate that the miR-124/PTBP1/PKM signaling axis plays a role in the reprogramming of cell metabolism during PE, leading to pulmonary arterial intimal remodeling. However, further research is required to clarify the underlying mechanisms and pathways. These results indicate that targeting this miRNA or its associated molecules could represent a new treatment strategy for preventing the progression of PE to CTEPH.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1806/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1806/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1806/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1806/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experimental procedures and animal care protocol were approved by the Animal Ethics Committee of Fujian Medical University Institutional Animal Care and Use Committee (IACUC FJMU 2024-Y-0941) and adhered to the Guide for the Care and Use of Laboratory Animals (NIH, Bethesda, MD, USA).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543-603. [Crossref] [PubMed]
- Olson KA, Schell JC, Rutter J. Pyruvate and Metabolic Flexibility: Illuminating a Path Toward Selective Cancer Therapies. Trends Biochem Sci 2016;41:219-30. [Crossref] [PubMed]
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008;452:230-3. [Crossref] [PubMed]
- Clower CV, Chatterjee D, Wang Z, et al. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci U S A 2010;107:1894-9. [Crossref] [PubMed]
- Wang D, Zhang H, Li M, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res 2014;114:67-78. [Crossref] [PubMed]
- Taniguchi K, Sugito N, Kumazaki M, et al. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett 2015;363:17-27. [Crossref] [PubMed]
- Deng C, Wu D, Yang M, et al. The role of tissue factor and autophagy in pulmonary vascular remodeling in a rat model for chronic thromboembolic pulmonary hypertension. Respir Res 2016;17:65. [Crossref] [PubMed]
- Hendriks PM, Kauling RM, Geenen LW, et al. Role of the electrocardiogram in the risk stratification of pulmonary hypertension. Heart 2023;109:208-15. [Crossref] [PubMed]
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023;61:2200879. [Crossref] [PubMed]
- Rosenkranz S. 2022 ESC/ERS guidelines on the diagnostics and treatment of pulmonary hypertension : A focussed review. Herz 2023;48:23-30. [Crossref] [PubMed]
- Otani N, Watanabe R, Tomoe T, et al. Pathophysiology and Treatment of Chronic Thromboembolic Pulmonary Hypertension. Int J Mol Sci 2023;24:3979. [Crossref] [PubMed]
- Yang M, Deng C, Wu D, et al. The role of mononuclear cell tissue factor and inflammatory cytokines in patients with chronic thromboembolic pulmonary hypertension. J Thromb Thrombolysis 2016;42:38-45. [Crossref] [PubMed]
- Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep 2016;17:1721-30. [Crossref] [PubMed]
- Zhang H, Wang D, Li M, et al. Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 2017;136:2468-85. [Crossref] [PubMed]
- Mercier O, Arthur Ataam J, Langer NB, et al. Abnormal pulmonary endothelial cells may underlie the enigmatic pathogenesis of chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2017;36:305-14. [Crossref] [PubMed]
- Husain FA, Martin MJ, Mullenix PS, et al. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg 2003;185:485-91. [Crossref] [PubMed]
- Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 2012;18:5554-61. [Crossref] [PubMed]
- David CJ, Chen M, Assanah M, et al. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2010;463:364-8. [Crossref] [PubMed]
- Chen M, Liu H, Li Z, et al. Mechanism of PKM2 affecting cancer immunity and metabolism in Tumor Microenvironment. J Cancer 2021;12:3566-74. [Crossref] [PubMed]
- Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 2007;8:93-103. [Crossref] [PubMed]
- Caruso P, Dunmore BJ, Schlosser K, et al. Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 2017;136:2451-67. [Crossref] [PubMed]
- Chen F, Li X, Aquadro E, et al. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic Biol Med 2016;99:167-78. [Crossref] [PubMed]


