
Analysis of risk factors and development of predictive model for acute myocardial injury in patients with acute exacerbation of chronic obstructive pulmonary disease
Highlight box
Key findings
• We investigated risk factors of acute myocardial injury (AMI) in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
What is known and what is new?
• Previous research has revealed that AMI is related to unfavorable outcomes among patients with AECOPD.
• This study is the first to explore the clinical features and risk factors for AMI in AECOPD patients and develop a clinical risk assessment model.
What is the implication, and what should change now?
• It will be helpful for clinicians to identify the population of AECOPD who are at a high risk of AMI and conduct early interventional measures.
Introduction
Serum cardiac troponin I (cTnI) and T are unique to the cardiac muscles and are typically detected as myocardial injury (MI) biomarkers in clinical practice (1). MI has been linked to adverse outcomes, such as increased mortality, prolonged hospitalization, and readmission within 30 days in various diseases. A prospective cohort study by Biasco et al. showed that the risks of death, intensive care unit (ICU) admission, and mechanical ventilation at 28 days were higher in hospitalized patients with influenza compared to those without MI (2). De Michieli et al. reported a significant association between MI and an increased incidence of poor short-term prognosis in coronavirus disease 2019 (COVID-19) patients, including the occurrence of severe respiratory or cardiac complications, as well as death during hospitalization or within 30 days after discharge (3).
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory pulmonary disease characterized by persistent respiratory symptoms and airflow limitation (4). Acute exacerbation of COPD (AECOPD) is a significant event marked by the worsening of respiratory symptoms within 14 days in COPD patients (5). AECOPD can lead to deterioration of lung function, severe respiratory symptoms, and multiple complications that significantly affect the quality of life of patients and is the leading cause of hospitalization and death in COPD patients (6). It is reported that cardiovascular diseases, such as MI, are common complications in the relatively short period following acute exacerbation of COPD (7). Previous researches have found that many patients hospitalized for AECOPD have elevated serum cardiac troponin (cTn) levels (8,9). According to a systematic review, the prevalence of MI during AECOPD is as high as above 51% (10). However, chronic MI is common in patients with COPD, and acute myocardial injury (AMI) is a poor outcome of AECOPD due to various factors. Unfortunately, compared with the AECOPD patients with chronic MI or non-MI, those with AMI have a worse prognosis, including a higher risk of recurrent hospitalization, heart failure, and all-cause mortality during follow-up (1,8,11,12). However, AMI in AECOPD patients is often underdiagnosed due to the lack of apparent myocardial ischemia symptoms and non-specific findings in the electrocardiogram (ECG). There are also few studies on the risk factors for AMI in AECOPD patients. Therefore, identifying modifiable risk factors and causes of AMI may lead to better treatment and prevention of adverse events in patients with AECOPD.
The relevance of a tailored risk assessment tool, such as a risk nomogram, for predicting AMI in AECOPD patients lies in its potential to integrate various patient-specific variables to estimate an individual’s risk. This approach offers a more detailed understanding of risk beyond what generalized guidelines can provide, facilitating personalized medical decision-making. Furthermore, early identification of patients at high risk of AMI could allow for timely interventions, potentially improving clinical outcomes and reducing hospitalization costs. This study was designed to explore the clinical features and risk factors associated with AMI among patients with AECOPD. A multivariable prediction model has also been developed. It provides clinical assistance for the early identification of AMI and the timely removal or avoidance of modifiable etiologies. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1992/rc).
Methods
Study population
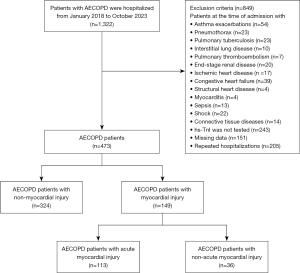
In this retrospective cohort study, 437 patients with AECOPD were hospitalized at the Department of Respiratory and Critical Care Medicine, Zhongnan Hospital of Wuhan University. They were enrolled from January 2018 to October 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2024001K), and written informed consent was obtained from all patients. The screening process for the 437 patients in this study is depicted in Figure 1.
Definitions
The diagnosis of COPD is according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2025 Report (13). The requisite condition for diagnosing COPD is irreversible airflow limitation: forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ratio <0.7 based on the post-bronchodilator. The AECOPD patients were identified by (I) a history of COPD and (II) worsening of respiratory symptoms such as dyspnea, cough, or sputum within 14 days (5). MI is defined as elevated levels of cardiac biomarkers, specifically, high-sensitivity cardiac troponin I (hs-TnI), such that they exceed the 99th percentile upper reference limit (URL) (14). At Zhongnan Hospital of Wuhan University, according to the laboratory standard, patients are diagnosed with MI when their hs-TnI levels are greater than 26.2 ng/mL. Based on the International Federation of Clinical Chemistry and Laboratory Medicine’s Task Force on Clinical Applications of Bio-Markers, AMI can be identified when the initial cTn value exceeds the 99th percentile of the URL (15). Specifically, it requires an increase of at least 50% of the 99th percentile value or a change greater than 20% relative to the initial value (16). Ischemic ECG changes were grouped by Minnesota coding, including ST-segment depression, T-wave inversion, or pathological Q-waves (17). FEV1 percent of predicted (highest obtained value) based on the post-bronchodilator was used to assess the severity of airflow limitation and further divided into grades 1–4 according to GOLD 2025 Report (13).
Inclusion and exclusion criteria
The inclusion criteria were: (I) patients with AECOPD needing hospitalization; (II) age ≥40 years. The exclusion criteria were as follows: (I) patients at the time of admission with asthma exacerbations, pneumothorax, pulmonary tuberculosis, interstitial lung disease, pulmonary thromboembolism, end-stage renal disease, ischemic heart disease, congestive heart failure, structural heart disease, myocarditis, sepsis, shock, and connective tissue diseases; (II) hs-TnI was not tested; (III) missing baseline data or lung function test. The data for patients with multiple hospitalizations was recorded from their most recent hospital stay. The included patients were divided into two groups based on MI: non-MI and AMI groups.
Data collection
The data were collected from the electronic medical record system, including demographic information, respiratory intensive care unit (RICU) admission status and in-hospital mortality outcomes, smoking history, past medical history, the use of cardiac medications (including calcium channel blockers, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, diuretics, statins, and antiplatelet agents) and inhaled corticosteroids (ICS), comorbidities, ischemic ECG changes, blood routine examination results, blood biochemical indicators, the systemic immune-inflammation index (SII), the systemic inflammatory response index (SIRI), neutrophil-to-lymphocyte ratio (NLR). SII and SIRI were calculated from the blood count using the following formulas: SII = platelet count × NLR and SIRI = monocyte count × NLR.
Statistical analysis
Continuous variables with a normal distribution were presented as mean ± standard deviation (SD) and compared using the t-test. Non-normally distributed continuous variables were expressed as median and interquartile range [median (Q1, Q3)], and their differences were analyzed using the Kruskal-Wallis test. Categorical variables were summarized as counts (percentage), and the Chi-squared or Fisher’s exact test was conducted to examine them. In addition, 437 patients with AECOPD were divided into four subgroups based on the quartiles of hs-TnI levels. Cochran-Armitage test for trend was used to explore the correlations between hs-TnI levels and hypercapnic respiratory failure (HRF), admission to the RICU, and in-hospital mortality. The preliminary risk factors of AMI in AECOPD patients were evaluated using univariate logistic regression analysis. The variables with P values less than 0.1 were candidates for the least absolute shrinkage and selection operator (LASSO) analysis. LASSO regression with tenfold cross-validation was also applied to reduce correlated variables and prevent overfitting in the subsequently generated nomogram. We used the LASSO regression analysis to select the most critical risk factors. The nomogram was developed to identify the presence of AMI based on multivariable logistic regression analysis. Its discrimination was evaluated using the receiver operating characteristic (ROC) curves, with internal validation performed using 1,000 bootstrapping resamples. Model calibration was assessed using a calibration curve and the Hosmer-Lemeshow goodness of fit test, while the clinical utility of the nomogram was evaluated through decision curve analysis (DCA). P<0.05 was considered statistically significant. Statistical analysis was conducted using R software (version 4.3.2). Multiple imputation was performed using the “mice package” for the missing data.
Results
Baseline characteristics of patients
A total of 437 patients with AECOPD were enrolled in the study, including 113 patients with AMI and 324 patients with non-MI. The general characteristics of all included patients (414 male and 23 female) are shown in Table 1. The mean age of the patients was 69.89±8.90 years. In comparison with the non-MI group, the AMI group had an older age (73.53 vs. 68.61 years, P<0.001), faster heart rate (97.00 vs. 81.00 bpm, P<0.001), and a higher prevalence of HRF (48.70% vs. 16.70%, P<0.001). The proportion of COPD patients with GOLD grade 3–4 was higher in the AMI group (89.6% vs. 62.4%, P<0.001). Regarding comorbidities, hypertension (48.70% vs. 35.8%, P=0.02) and arrhythmia (43.4% vs. 32.7%, P=0.06) were more common in the AMI group. The occurrence of ischemic ECG changes was also more significant in the AMI group than in the non-MI group. When it comes to drug usage, there was a significant difference in the utilization of cardiac medications between the two groups (61.1% vs. 37.7%, P<0.001), while the situation was the opposite for ICS. Moreover, the admission rate to the RICU and the in-hospital mortality rate in the AMI group are substantially higher than those in the non-MI group. The Cochran-Armitage trend test indicated that elevated hs-TnI levels were associated with higher risks of in-hospital death, RICU admission, and HRF (Z1 =−4.20, Z2 =−4.51, Z3 =−6.44, P<0.001).
Table 1
| Characteristics | Total patients (n=437) | AECOPD patients | P | |
|---|---|---|---|---|
| Non-myocardial injury (n=324) | Acute myocardial injury (n=113) | |||
| Gender | 0.79 | |||
| Male | 414 (94.7) | 308 (95.1) | 106 (93.8) | |
| Female | 23 (5.3) | 16 (4.9) | 7 (6.2) | |
| Age (years) | 69.89±8.90 | 68.61±8.39 | 73.53±9.36 | <0.001 |
| HR (bpm) | 82.00 (72.00, 96.00) | 81.00 (71.00, 91.00) | 97.00 (81.00, 114.50) | <0.001 |
| Smoking (years) | 30.00 (30.00, 40.00) | 30.00 (30.00, 40.00) | 30.00 (25.00, 41.50) | 0.94 |
| HRF | 109 (24.9) | 54 (16.7) | 55 (48.7) | <0.001 |
| GOLD grades | <0.001 | |||
| GOLD 1 | 23 (7.0) | 22 (8.4) | 1 (1.5) | |
| GOLD 2 | 83 (25.2) | 77 (29.3) | 6 (9.0) | |
| GOLD 3–4 | 224 (67.9) | 164 (62.4) | 60 (89.6) | |
| Comorbidities | ||||
| Hypertension | 171 (39.1) | 116 (35.8) | 55 (48.7) | 0.02 |
| Diabetes | 45 (10.3) | 29 (9.0) | 16 (14.2) | 0.17 |
| Arrhythmia | 155 (35.5) | 106 (32.7) | 49 (43.4) | 0.06 |
| Pneumonia | 131 (30.0) | 100 (30.9) | 31 (27.4) | 0.57 |
| Past medical history | ||||
| CAD | 58 (13.3) | 39 (12.0) | 19 (16.8) | 0.26 |
| Stroke | 27 (6.2) | 20 (6.2) | 7 (6.2) | >0.99 |
| Cancer | 33 (7.6) | 20 (6.2) | 13 (11.5) | 0.10 |
| Cardiac medications | 191 (43.7) | 122 (37.7) | 69 (61.1) | <0.001 |
| ICS | 190 (43.5) | 146 (45.1) | 44 (38.9) | 0.31 |
| ECG changes | 165 (37.8) | 95 (29.3) | 70 (61.9) | <0.001 |
| RICU | 22 (5.0) | 4 (1.2) | 18 (15.9) | <0.001 |
| In-hospital death | 12 (2.7) | 2 (0.6) | 10 (8.8) | <0.001 |
Data are presented as n (%), mean ± standard deviation or median (Q1, Q3). AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CAD, coronary artery disease; ECG, electrocardiogram; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, heart rate; HRF, hypercapnic respiratory failure; ICS, inhaled corticosteroids; RICU, respiratory intensive care unit.
Differences in laboratory findings in patients with AECOPD
The laboratory findings of patients with AECOPD are presented in Table 2. In the AMI group, there were higher levels of inflammatory parameters (SII, SIRI, and NLR), neutrophil count, white blood cell count (WBC), and red blood cell distribution width (RDW) (P<0.001), but lower lymphocyte count, eosinophil count and platelets in the blood (P<0.05). Furthermore, compared to the non-MI group, the patients who developed AMI had increased levels of aspartate aminotransferase (AST), random blood glucose (RBG), blood urea nitrogen (BUN), creatinine, cystatin C, fibrinogen and D-dimer in plasma (P<0.001). Meanwhile, serum albumin, calcium, and chloride ions were significantly lower in the AMI group (P<0.001).
Table 2
| Laboratory findings | Total patients (n=437) | AECOPD patients | P | |
|---|---|---|---|---|
| Non-myocardial injury (n=324) | Acute myocardial injury (n=113) | |||
| Composite inflammatory index | ||||
| SII | 707.72 (414.18, 1,532.47) | 608.50 (375.77, 1,047.66) | 1,660.82 (668.32, 3,778.17) | <0.001 |
| SIRI | 2.20 (1.17, 5.30) | 1.73 (1.04, 3.09) | 5.72 (2.33, 13.79) | <0.001 |
| NLR | 3.64 (2.33, 7.49) | 3.19 (2.05, 5.56) | 9.53 (3.85, 23.24) | <0.001 |
| WBC (×109 cells/L) | 6.74 (5.46, 9.00) | 6.38 (5.23, 7.80) | 8.88 (6.69, 13.40) | <0.001 |
| RBC (×1012 cells/L) | 4.24 (3.86, 4.59) | 4.26 (3.95, 4.59) | 4.15 (3.72, 4.58) | 0.07 |
| PLT (×109 cells/L) | 186.00 (147.00, 231.00) | 190.00 (157.00, 231.00) | 166.00 (122.00, 234.00) | 0.02 |
| HGB (g/L) | 129.70 (120.00, 140.00) | 131.00 (123.00, 141.00) | 125.00 (108.00, 138.00) | 0.001 |
| NEUT (×109 cells/L) | 4.58 (3.30, 6.72) | 4.10 (3.10, 5.43) | 7.00 (4.58, 11.40) | <0.001 |
| LYM (×109 cells/L) | 1.14 (0.80, 1.60) | 1.23 (0.94, 1.69) | 0.78 (0.40, 1.20) | <0.001 |
| MO (×109 cells/L) | 0.58 (0.42, 0.73) | 0.56 (0.41, 0.70) | 0.60 (0.43, 0.91) | 0.12 |
| EO (×109 cells/L) | 0.10 (0.03, 0.20) | 0.12 (0.08, 0.21) | 0.04 (0.00, 0.11) | <0.001 |
| HCT (%) | 39.50 (36.30, 42.50) | 39.60 (36.90, 42.52) | 38.50 (32.40, 42.30) | 0.02 |
| RDW (%) | 13.90 (13.20, 14.60) | 13.80 (13.20, 14.40) | 14.20 (13.40, 15.10) | <0.001 |
| ALT (U/L) | 16.00 (12.00, 24.00) | 15.00 (11.00, 22.00) | 18.00 (12.00, 29.50) | 0.01 |
| AST (U/L) | 20.00 (16.00, 27.00) | 19.00 (16.00, 25.00) | 25.00 (20.00, 36.00) | <0.001 |
| ALB (g/L) | 35.48±4.58 | 36.15±4.42 | 33.55±4.51 | <0.001 |
| RBG (mmol/L) | 5.10 (4.54, 6.67) | 4.92 (4.49, 5.80) | 6.52 (5.09, 9.14) | <0.001 |
| BUN (mmol/L) | 6.13 (4.80, 7.70) | 5.65 (4.57, 7.00) | 8.20 (6.35, 10.37) | <0.001 |
| Creatinine (μmol/L) | 75.70 (65.05, 88.40) | 74.70 (64.65, 84.45) | 84.55 (68.95, 107.60) | <0.001 |
| Uric acid (μmol/L) | 326.00 (259.92, 408.42) | 317.40 (258.03, 398.35) | 355.45 (268.05, 420.68) | 0.03 |
| Cystatin C (mg/L) | 1.04 (0.89, 1.29) | 1.00 (0.85, 1.15) | 1.40 (1.07, 1.85) | <0.001 |
| Serum potassium (mmol/L) | 3.87 (3.63, 4.14) | 3.85 (3.60, 4.10) | 3.99 (3.68, 4.40) | 0.01 |
| Serum sodium (mmol/L) | 140.60 (138.00, 142.33) | 140.60 (138.48, 142.40) | 140.10 (137.00, 142.02) | 0.14 |
| Serum chloride (mmol/L) | 103.20 (99.50, 106.00) | 104.10 (101.10, 106.60) | 100.00 (96.45, 103.38) | <0.001 |
| Serum calcium (mmol/L) | 2.16 (2.08, 2.25) | 2.18 (2.10, 2.25) | 2.09 (2.01, 2.22) | <0.001 |
| Serum magnesium (mmol/L) | 0.87 (0.81, 0.93) | 0.87 (0.81, 0.93) | 0.85 (0.79, 0.95) | 0.23 |
| Fibrinogen (mg/dL) | 363.00 (298.00, 431.00) | 352.00 (287.75, 425.25) | 406.00 (342.00, 450.25) | <0.001 |
| D-dimer (ng/mL) | 200.00 (114.00, 380.50) | 160.00 (96.00, 265.25) | 427.00 (249.50, 937.50) | <0.001 |
Data are presented as median (Q1, Q3) or mean ± standard deviation. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; EO, eosinophil count; HCT, hematocrit; HGB, hemoglobin; LYM, lymphocyte count; MO, monocyte count; NEUT, neutrophil count; NLR, neutrophil-to-lymphocyte ratio; PLT, platelets; RBG, random blood glucose; RBC, red blood cells count; RDW, red blood cell distribution width; SII, systemic immune-inflammation index; SIRI, system inflammatory response index; WBC, white blood cells count.
Variable selection and nomogram variable screening
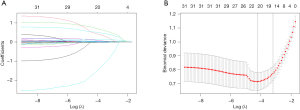
The results of univariable logistic regression for AMI are shown in Table 3. The variables with statistical significance (P<0.1) were incorporated into the LASSO regression analysis following univariable analysis. Twenty-one non-zero coefficient variables were identified as potential risk factors according to the minimum criteria in the LASSO regression analysis (Figure 2A,2B). There were age, albumin, AST, alanine aminotransferase (ALT), BUN, cardiac medications, ECG changes, GOLD 3–4, creatinine, cystatin C, heart rate, HRF, hemoglobin, hematocrit, NLR, SIRI, RBG, RDW, serum calcium ions, serum chloride ions, and fibrinogen. Subsequently, we included the 21 potential risk factors in the multivariate logistic regression analysis (Table 3). Finally, seven final and independent risk factors for the AECOPD with AMI were included in the model after multivariate logistic regression, including cardiac medications, ECG changes, HRF, age, heart rate, BUN, and serum calcium ions.
Table 3
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Gender | 0.7866 (0.3262–2.0942) | 0.61 | – | – | |
| Age | 1.0682 (1.0410–1.0973) | <0.001 | 1.0309 (0.9859–1.0779) | 0.18 | |
| HR | 1.0457 (1.0331–1.0593) | <0.001 | 1.0355 (1.009–1.0627) | 0.02 | |
| Smoking | 0.9999 (0.9871–1.0132) | 0.99 | – | – | |
| GOLD 3–4 | 1.5694 (0.9919–2.5278) | 0.06 | 0.9197 (0.2362–3.5808) | 0.91 | |
| Cardiac medications | 2.5965 (1.6786–4.0522) | <0.001 | 2.0277 (1.0395–3.9554) | 0.04 | |
| ICS | 0.7774 (0.5000–1.1998) | 0.26 | – | – | |
| ECG changes | 4.3344 (2.7697–6.8694) | <0.001 | 2.8836 (1.475–5.6373) | 0.002 | |
| HRF | 4.1975 (2.7245–6.5118) | <0.001 | 2.2522 (0.8403–6.0364) | 0.12 | |
| Hypertension | 1.7004 (1.1020–2.6247) | 0.02 | – | – | |
| Diabetes | 1.6779 (0.8572–3.1849) | 0.12 | – | – | |
| Arrhythmia | 1.7304 (1.1231–2.6698) | 0.01 | – | – | |
| CAD | 1.5715 (0.8599–2.8011) | 0.13 | – | – | |
| Pneumonia | 0.8598 (0.5314–1.3682) | 0.53 | – | – | |
| Stroke | 1.0038 (0.3847–2.3380) | 0.10 | – | – | |
| Cancer | 0.8691 (0.5751–1.0948) | 0.35 | – | – | |
| SII | 1.0003 (1.0002–1.0004) | <0.001 | – | – | |
| SIRI | 1.1248 (1.0837–1.1735) | <0.001 | 1.0261 (0.9620–1.0945) | 0.43 | |
| NLR | 1.0928 (1.0656–1.1244) | <0.001 | 1.0265 (0.9815–1.0736) | 0.25 | |
| WBC | 1.2001 (1.1349–1.2748) | <0.001 | – | – | |
| RBC | 0.7016 (0.4921–0.9923) | 0.05 | – | – | |
| HGB | 0.9785 (0.9667–0.9901) | <0.001 | 0.9979 (0.9773–1.019) | 0.85 | |
| PLT | 0.9976 (0.9945–1.0006) | 0.12 | – | – | |
| NEUT | 1.2310 (1.1609–1.3117) | <0.001 | – | – | |
| LYM | 1.0085 (0.9569–1.0542) | 0.68 | – | – | |
| MO | 1.2070 (0.8252–1.8778) | 0.32 | – | – | |
| EO | 0.0391 (0.0053–0.2160) | <0.001 | – | – | |
| HCT | 0.9639 (0.9414–0.9862) | 0.002 | 0.9695 (0.9321–1.0084) | 0.12 | |
| RDW | 1.2538 (1.0831–1.4577) | 0.002 | 1.2321 (0.9906–1.5323) | 0.06 | |
| ALT | 1.0185 (1.0087–1.0300) | <0.001 | 1.0096 (0.9919–1.0276) | 0.29 | |
| AST | 1.0434 (1.0272–1.0620) | <0.001 | 1.0073 (0.9786–1.0369) | 0.62 | |
| ALB | 0.8760 (0.8311–0.9210) | <0.001 | 0.9938 (0.9118–1.0832) | 0.89 | |
| RBG | 1.2253 (1.1304–1.3376) | <0.001 | 1.0718 (0.9539–1.2043) | 0.24 | |
| BUN | 1.4418 (1.3117–1.6004) | <0.001 | 1.1727 (1.0047–1.3686) | 0.044 | |
| Creatinine | 1.0200 (1.0121–1.0289) | <0.001 | 1.0088 (0.9943–1.0235) | 0.24 | |
| Uric acid | 1.0025 (1.0007–1.0043) | 0.01 | – | – | |
| Cystatin C | 2.3938 (1.4786–3.7908) | <0.001 | 1.0412 (0.8573–1.2646) | 0.69 | |
| Serum potassium | 2.3938 (1.5592–3.7364) | <0.001 | – | – | |
| Serum sodium | 0.9968 (0.9698–1.0312) | 0.81 | – | – | |
| Serum chloride | 0.9052 (0.8686–0.9416) | <0.001 | 0.9435 (0.8869–1.0038) | 0.07 | |
| Serum calcium | 0.0584 (0.0123–0.2340) | <0.001 | 0.0509 (0.0030–0.8642) | 0.040 | |
| Serum magnesium | 0.6801 (0.0883–5.1342) | 0.71 | – | – | |
| Fibrinogen | 1.0034 (1.0015–1.0055) | <0.001 | 1.0014 (0.9984–1.0043) | 0.36 | |
| D-dimer | 1.0004 (1.0002–1.0007) | 0.001 | – | – | |
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CAD, coronary artery disease; CI, confidence interval; ECG, electrocardiogram; EO, eosinophil count; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HCT, hematocrit; HGB, hemoglobin; HR, heart rate; HRF, hypercapnic respiratory failure; ICS, inhaled corticosteroids; LYM, lymphocyte count; MO, monocyte count; NEUT, neutrophil count; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PLT, platelets; RBG, random blood glucose; RBC, red blood cells count; RDW, red blood cell distribution width; SII, systemic immune-inflammation index; SIRI, system inflammatory response index; WBC, white blood cells count.
Nomogram construction and internal validation
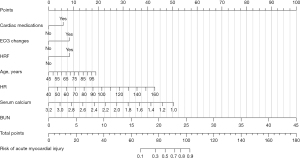
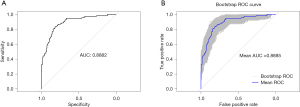
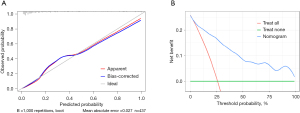
We further analyzed multivariable logistic regression and generated a prediction model to understand the relationship between AMI and these risk factors. The outcomes of the multiple logistic regression analyses are shown in Table 4. Based on our multivariate model, we have developed a nomogram for identifying the probability of AMI in AECOPD patients (Figure 3). The total scores of the nomogram were determined by the individual scores and used to obtain the probability of identifying the presence of AMI. The nomogram model demonstrated superior performance, with an area under the receiver operating characteristic curve (AUC) value of 0.8882 [95% confidence interval (CI): 0.8525–0.9238] (Figure 4A). The internally validated AUC of 1,000 bootstrap samples was 0.8885 (95% CI: 0.8874–0.8895) (Figure 4B). The visible calibration curve plot with 1,000 bootstraps indicated that the model was well-calibrated (Figure 5A). Similarly, the Hosmer-Lemeshow test had a nonsignificant P value of 0.16 (χ2=10.58), suggesting no statistical departure from a perfect fit between the predicted and observed values. Furthermore, the DCA curves showed that the model could bring more benefits for predicting AMI in AECOPD patients (Figure 5B).
Table 4
| Variables | Coefficient | OR (95% CI) | P |
|---|---|---|---|
| Age | 0.042 | 1.043 (1.006–1.081) | 0.02 |
| Cardiac medications | 0.711 | 2.036 (1.125–3.685) | 0.02 |
| ECG changes | 1.005 | 2.733 (1.526–4.894) | <0.001 |
| HRF | 1.030 | 2.801 (1.495–5.250) | 0.001 |
| HR | 0.044 | 1.044 (1.025–1.065) | <0.001 |
| BUN | 0.271 | 1.311 (1.170–1.470) | <0.001 |
| Serum calcium | −2.795 | 0.061 (0.008–0.485) | 0.009 |
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BUN, blood urea nitrogen; CI, confidence interval; ECG, electrocardiogram; HR, heart rate; HRF, hypercapnic respiratory failure; OR, odds ratio.
Discussion
It is reported that the risk of acute cardiovascular events is three to five times higher in the first year following a severe COPD exacerbation compared to non-exacerbation periods (18). Several studies have shown that elevated levels of myocardial-specific plasma troponin, a diagnostic criteria for MI, are associated with increased in-hospital death and all-cause mortality for COPD exacerbations (10,19). Consistent with previous studies, our results found that the frequency of RICU admissions and in-hospital deaths was higher in the AMI group compared with the non-MI group. Effectively identifying high-risk individuals with AMI is crucial for reducing the risk of poor outcomes in AECOPD patients. In this study, we used LASSO analysis to identify the independent risk factors for AMI, which helped to minimize the coefficients of complex clinical variables and eliminate those that were relatively unnecessary. A group of valid and concise variables was used to construct a nomogram for evaluating the risk of AMI in patients with AECOPD. The results showed that AECOPD patients with AMI were more likely to experience complications such as HRF, tachycardia, serum electrolyte imbalances, and even kidney injury. We found seven factors significantly linked to AMI in AECOPD: advanced age, use of cardiac medications, ischemic ECG changes, HRF, rapid heart rate, decreased serum calcium, and elevated levels of BUN. We also created a visualization of the risk assessment model using multivariable logistics regression analysis. The model showed a high AUC of 0.8882, indicating strong predictive ability, and it has been internally validated.
Our research indicates that older age and increased heart rate are important risk factors for AMI in patients with AECOPD. With aging, some factors that induce or cause AMI usually emerge, such as arterial aging, age-related atherosclerosis, the reduction of coronary artery reserve, and the occurrence of comorbidities (for example, chronic anemia, arrhythmia, infection, etc.) (20). Obas V et al. have shown that aging contributes to structural and functional changes in various parts of the heart and vasculature, which can increase the risk of cardiovascular diseases (21). The prevalence of serum cTn elevation in the setting of tachyarrhythmia is generally estimated to be between 30% and 50%. Serum cTn elevation in the setting of tachyarrhythmia is often an indicator of MI and a supply-demand mismatch from increased energy demands rather than coronary artery obstruction or myocardial necrosis (22). A rapid heart rate could increase the myocardial workload, shorten the diastolic duration, impede coronary perfusion, and lead to an imbalance between oxygen supply and demand, resulting in myocardial damage (23). Therefore, detecting and controlling the heart rate within a certain range is beneficial.
The use of cardiac drugs in patients with COPD reflects the prevalence of their cardiovascular diseases. Cardiovascular disease is a common comorbidity in COPD patients, such as coronary artery disease (CAD), hypertension, and atrial fibrillation. In the AMI group, the proportions of patients with hypertension and arrhythmia were significantly higher than those in the non-MI group. Hypertension is one of the leading causes of cardiovascular disease, including CAD, heart failure, chronic kidney disease, peripheral vascular disease, and stroke (24). Hypertensive heart disease is characterized by coronary microvascular dysfunction, which involves endothelial dysfunction, smooth muscle cell dysfunction, and dysregulation of sympathetic innervation. These factors significantly contribute to myocardial ischemia (14). A 12-lead ECG is a rapid, convenient, and widely accessible diagnostic tool for detecting ischemic ECG abnormalities. However, ischemic ECG changes are relatively common in COPD patients and are influenced by various factors. Continuous monitoring and comprehensive assessment are required to determine whether there is an MI or myocardial infarction. Indeed, the death risk of COPD patients with ischemic ECG changes increases significantly, consistent with previous studies (17,25).
Recently, a population-based longitudinal study found that hospitalized patients whose potential cause is COPD accompanied by HRF indicate a significant risk of readmission, especially among the elderly (26). In our study, there was a higher proportion of patients with HRF in the AMI group. For AECOPD patients with HRF, noninvasive ventilation (NIV) is a commonly used and effective treatment method. However, NIV also has some adverse effects on the heart. During the treatment process, dyspnea, anxiety, and discomfort arising from inadequate NIV support can trigger the release of stress-related catecholamines. Subsequently, this leads to an increase in myocardial oxygen demands and a heightened risk of dysrhythmias (26). Furthermore, oxygen delivery via coronary blood vessels may be hampered due to insufficient gas exchange from lung injury and low mixed venous partial pressure of oxygen (PaO2) in arterial blood caused by high oxygen consumption of inspiratory muscles (27).
The elevation of creatinine and BUN in the short term may indicate renal insufficiency and even acute kidney injury (AKI). It has been reported that patients with AECOPD who have CAD, anemia, and have received mechanical ventilation therapy are at a higher risk for AKI, and patients with AKI are more likely to experience in-hospital mortality, require mechanical ventilation and ICU admission and have a longer length of stay (28). In our study, we found that the risk factors for AKI were also prevalent in the AECOPD with AMI group. When kidney function is impaired, there is a significant increase in oxidative stress and inflammation of the myocardium. Moreover, impaired excretion of toxins also can directly cause AMI (29,30). It has been stated that there are common pathways linking cardio-renal and reno-cardiac interactions in the pathophysiology. In AECOPD patients with AKI, the accumulation of toxic metabolites, activation of cellular immunity and inflammation, dysregulation of the neuro-hormonal axis, changes in metabolism and hemodynamics, and altered acid-base or fluid status may collectively contribute to myocardial damage (31). Studies have explored the association between low serum calcium levels and the increased risk of cardiovascular mortality (32-35). A large prospective cohort study based on general populations reported that the hazard ratio for cardiovascular disease mortality is 0.75 for every 0.1 mmol/L decrease in serum calcium when the level is below 2.35 mmol/L (32). AECOPD patients are frequently complicated with renal insufficiency, heart dysfunction, and malnutrition, which makes it more likely for them to have low calcium levels and increases the risk of AMI.
However, there are some limitations in this study. Firstly, it was conducted at a single center with a relatively small sample size, and only internal validation was performed. The applicability of the results to other regions and races needs to be cautiously approached. External validation requires more data from other multi-center studies, and it will be crucial to expand the sample size and conduct multi-center studies in the future. Secondly, critical clinical data such as PaO2, partial pressure of carbon dioxide (PaCO2) in arterial blood, and complete lung function were excluded due to the retrospective design and a large amount of data loss, which may have caused potential bias in some variables in this study. It is recommended that alternative techniques, such as impulse oscillometry, be used in the future to validate these frail COPD patients. Thirdly, the absence of post-discharge cardiac evaluation such as echocardiography, coronary angiography, and cardiac magnetic resonance imaging (MRI) and major adverse cardiovascular events (MACE) data limits our ability to determine whether AMI in AECOPD reflects transient stress or underlying structural heart disease. Therefore, future prospective studies, including cardiac follow-up evaluation (e.g., serial echocardiography, coronary angiography, cardiac MRI) and MACE surveillance, are needed to validate our predictive model and clarify the clinical trajectory of these high-risk COPD patients.
Conclusions
In summary, we have found seven independent risk factors of AMI in AECOPD. Advanced age, usage of cardiac medications, HRF, serum electrolyte imbalances, fast heart rate, ischemia ECG, and kidney injury may be the underlying etiologies that necessitate immediate attention.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1992/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1992/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1992/prf
Funding: This study was supported by grants from Zhongnan Hospital of Wuhan University Science, Technology and Innovation Cultivation Fund (grant No. CXPY2022084).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1992/coif). W.W. was supported by grants from Zhongnan Hospital of Wuhan University Science, Technology and Innovation Cultivation Fund (grant No. CXPY2022084). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2024001K), and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elmenawi KA, Anil V, Gosal H, et al. The Importance of Measuring Troponin in Chronic Obstructive Pulmonary Disease Exacerbations: A Systematic Review. Cureus 2021;13:e17451. [Crossref] [PubMed]
- Biasco L, Valotta A, Klersy C, et al. Association among myocardial injury and mortality in Influenza: A prospective cohort study. Int J Cardiol 2022;369:48-53. [Crossref] [PubMed]
- De Michieli L, Ola O, Knott JD, et al. High-Sensitivity Cardiac Troponin T for the Detection of Myocardial Injury and Risk Stratification in COVID-19. Clin Chem 2021;67:1080-9. [Crossref] [PubMed]
- Celli B, Fabbri L, Criner G, et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am J Respir Crit Care Med 2022;206:1317-25. [Crossref] [PubMed]
- Celli BR, Fabbri LM, Aaron SD, et al. An Updated Definition and Severity Classification of Chronic Obstructive Pulmonary Disease Exacerbations: The Rome Proposal. Am J Respir Crit Care Med 2021;204:1251-8. [Crossref] [PubMed]
- Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med 2020;73:1-6. [Crossref] [PubMed]
- Müllerová H, Marshall J, de Nigris E, et al. Association of COPD exacerbations and acute cardiovascular events: a systematic review and meta-analysis. Ther Adv Respir Dis 2022;16:17534666221113647. [Crossref] [PubMed]
- Laribi S, Pemberton CJ, Kirwan L, et al. Mortality and acute exacerbation of COPD: a pilot study on the influence of myocardial injury. Eur Respir J 2017;49:1700096. [Crossref] [PubMed]
- Shafuddin E, Chang CL, Cooray M, et al. Changes in biomarkers of cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Respir Med 2018;145:192-9. [Crossref] [PubMed]
- Pavasini R, d'Ascenzo F, Campo G, et al. Cardiac troponin elevation predicts all-cause mortality in patients with acute exacerbation of chronic obstructive pulmonary disease: Systematic review and meta-analysis. Int J Cardiol 2015;191:187-93. [Crossref] [PubMed]
- Kallis C, Kaura A, Samuel NA, et al. The Relationship Between Cardiac Troponin in People Hospitalised for Exacerbation of COPD and Major Adverse Cardiac Events (MACE) and COPD Readmissions. Int J Chron Obstruct Pulmon Dis 2023;18:2405-16. [Crossref] [PubMed]
- Kadesjö E, Roos A, Siddiqui A, et al. Acute versus chronic myocardial injury and long-term outcomes. Heart 2019;105:1905-12. [Crossref] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis Management and Prevention of Chronic Obstructive Pulmonary Disease 2025 Report. Available online: https://goldcopd.org/archived-reports
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018;138:e618-51. [Crossref] [PubMed]
- Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645-55. [Crossref] [PubMed]
- McCarthy CP, Raber I, Chapman AR, et al. Myocardial Injury in the Era of High-Sensitivity Cardiac Troponin Assays: A Practical Approach for Clinicians. JAMA Cardiol 2019;4:1034-42. [Crossref] [PubMed]
- Nilsson U, Mills NL, McAllister DA, et al. Cardiac biomarkers of prognostic importance in chronic obstructive pulmonary disease. Respir Res 2020;21:162. [Crossref] [PubMed]
- Nordon C, Rhodes K, Quint JK, et al. EXAcerbations of COPD and their OutcomeS on CardioVascular diseases (EXACOS-CV) Programme: protocol of multicountry observational cohort studies. BMJ Open 2023;13:e070022. [Crossref] [PubMed]
- Søyseth V, Kononova N, Neukamm A, et al. Systemic inflammation induced by exacerbation of COPD or pneumonia in patients with COPD induces cardiac troponin elevation. BMJ Open Respir Res 2021;8:e000997. [Crossref] [PubMed]
- Putot A, Putot S, Chagué F, et al. New horizons in Type 2 myocardial infarction: pathogenesis, assessment and management of an emerging geriatric disease. Age Ageing 2022;51:afac085. [Crossref] [PubMed]
- Obas V, Vasan RS. The aging heart. Clin Sci (Lond) 2018;132:1367-82. [Crossref] [PubMed]
- Maayah M, Grubman S, Allen S, et al. Clinical Interpretation of Serum Troponin in the Era of High-Sensitivity Testing. Diagnostics (Basel) 2024;14:503. [Crossref] [PubMed]
- Keefe JA, Garber R, McCauley MD, et al. Tachycardia and Atrial Fibrillation-Related Cardiomyopathies: Potential Mechanisms and Current Therapies. JACC Heart Fail 2024;12:605-15. [Crossref] [PubMed]
- Zhang S, Liu L, Huang YQ, et al. The association between serum uric acid levels and ischemic stroke in essential hypertension patients. Postgrad Med 2020;132:551-8. [Crossref] [PubMed]
- Nilsson U, Blomberg A, Johansson B, et al. Ischemic ECG abnormalities are associated with an increased risk for death among subjects with COPD, also among those without known heart disease. Int J Chron Obstruct Pulmon Dis 2017;12:2507-14. [Crossref] [PubMed]
- Chung Y, Garden FL, Marks GB, et al. Long-term cohort study of patients presenting with hypercapnic respiratory failure. BMJ Open Respir Res 2024;11:e002266. [Crossref] [PubMed]
- MacIntyre NR. Physiologic Effects of Noninvasive Ventilation. Respir Care 2019;64:617-28. [Crossref] [PubMed]
- Wan X, Chen D, Tan Y, et al. Incidence, Risk Factors, and Prognostic Implications of Acute Kidney Injury in Patients with Acute Exacerbation of COPD. Int J Chron Obstruct Pulmon Dis 2020;15:1085-92. [Crossref] [PubMed]
- Hong J, Chatila KF, John JJ, et al. Insight on the Etiologies of Chronically Elevated Troponin. Curr Probl Cardiol 2023;48:101204. [Crossref] [PubMed]
- Chen TH, Yang YC, Wang JC, et al. Curcumin treatment protects against renal ischemia and reperfusion injury-induced cardiac dysfunction and myocardial injury. Transplant Proc 2013;45:3546-9. [Crossref] [PubMed]
- Schefold JC, Filippatos G, Hasenfuss G, et al. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 2016;12:610-23. [Crossref] [PubMed]
- Hou X, Hu J, Liu Z, et al. L-shaped association of serum calcium with all-cause and CVD mortality in the US adults: A population-based prospective cohort study. Front Nutr 2022;9:1097488. [Crossref] [PubMed]
- Schmitz T, Thilo C, Linseisen J, et al. Low serum calcium is associated with higher long-term mortality in myocardial infarction patients from a population-based registry. Sci Rep 2021;11:2476. [Crossref] [PubMed]
- Kobylecki CJ, Nordestgaard BG, Afzal S. Plasma Ionized Calcium and Risk of Cardiovascular Disease: 106 774 Individuals from the Copenhagen General Population Study. Clin Chem 2021;67:265-75. [Crossref] [PubMed]
- Jensen AC, Polcwiartek C, Søgaard P, et al. The Association Between Serum Calcium Levels and Short-Term Mortality in Patients with Chronic Heart Failure. Am J Med 2019;132:200-208.e1. [Crossref] [PubMed]


