
SMARCA4-deficient undifferentiated thoracic tumor: a case report and literature review
Highlight box
Key findings
• The first-line combined regimen containing immunotherapy plus antiangiogenic therapy and chemotherapy demonstrated durable efficacy in SMARCA4-deficient undifferentiated thoracic tumor (SMARCA4-UT).
What is known and what is new?
• SMARCA4-UT exhibits aggressive growth, is associated with a poor prognosis, and is usually unresponsive to cytotoxic chemotherapy. Immunotherapy has shown efficacy in these patients in a few recent studies.
• We report the first case of a patient with SMARCA4-UT who was treated with a combined first-line regimen consisting of immunotherapy plus antiangiogenic therapy and chemotherapy. Treatment was well tolerated and resulted in durable response.
What is the implication, and what should change now?
• The combined regimen containing immunotherapy plus antiangiogenic therapy and chemotherapy could be a novel treatment option for patients with SMARCA4-UT. Further prospective studies are needed to validate the efficacy and safety of this regimen.
Introduction
Background
SMARCA4-deficient undifferentiated thoracic tumor (SMARCA4-UT) is a high-grade malignant tumor, which is histologically characterized by the presence of an undifferentiated or rhabdoid phenotype (1). It is more likely to develop in heavy smokers and is associated with smoking-related genetic changes. Approximately 5–10% of classical non-small cell lung cancer (NSCLC) cases and a subset of those with thyroid transcription factor 1 (TTF-1)-negative neuroendocrine carcinomas (including large-cell neuroendocrine carcinoma and small-cell carcinoma) exhibit a loss of SMARCA4 expression (2,3). Notably, the latest World Health Organization classification specifically distinguishes SMARCA4-deficient NSCLC from the distinct entity designated as SMARCA4-UT, with the latter being categorized within the group of “other lung epithelial tumors” (4). SMARCA4-UT is typically advanced at the time of diagnosis, with widespread involvement of thoracic structures, and metastasizes to lymph nodes, bone, and adrenal glands (1,5). SMARCA4-UT commonly exhibits aggressive growth, involves a poor prognosis, and is unresponsive to cytotoxic chemotherapy. Currently, no guidelines exist regarding the standard treatment of this disease.
Study rationale
Published reports have demonstrated the efficacy of immunotherapy in patients with SMARCA4-UT (6-9). Similarly, a recent study reported three cases of SMARCA4-deficient thoracic sarcoma (SMARCA4-DTS) treated with atezolizumab plus bevacizumab, paclitaxel, and carboplatin (ABCP) as the first-line therapy (10). Partial response was observed in all three patients, and the first-line ABCP regimen for SMARCA4-DTS showed durable efficacy regardless of programmed death-ligand 1 (PD-L1) expression.
Objective
Here, we present a case of a patient with stage IV SMARCA4-UT who received a regimen consisting of camrelizumab, bevacizumab, pemetrexed, and carboplatin and discuss the therapeutic strategies for SMARCA4-UT. We present this case in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-541/rc).
Case presentation
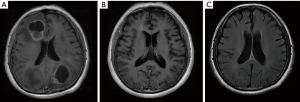
A 71-year-old Chinese male was admitted to Tieling Central Hospital with cough and shortness of breath in November 2021. The patient underwent subtotal laryngectomy in August 2015, and the postoperative pathology was poorly differentiated squamous cell carcinoma. Without being subjected to radiotherapy or chemotherapy after surgery, the disease appeared stable on regular reexamination. The patient did not have a history of hypertension or diabetes but a family history of cancer with his father and mother suffering from pancreatic and gastric cancer, respectively, as well as his sister with liver cancer. He had a history of heavy smoking of 20 pack-years and quit smoking more than 9 years ago. Contrast-enhanced chest computed tomography (CT) scan revealed a 58.3 mm × 56.2 mm mass in the right lower lobe of the lung and pleural effusion in the left thoracic cavity. The liver apex showed a round, slightly hypodense shadow of 21.1 mm × 21.1 mm in size. The 18F-fluorodeoxyglucose positron emission tomography-CT (18F-FDG-PET-CT) examination corroborated the mass in the lower lobe anterior basal segment of the right lung (Figure 1A), measuring approximately 6.1 cm × 5.1 cm, with a maximum standard uptake value (SUVmax) of 21.9, suggesting malignancy. The nodule with high FDG metabolism in the left lobe of the liver measured approximately 1.9 cm × 1.8 cm, with a SUVmax of 11.51 and was considered most likely to be metastatic disease (Figure 1B). There was a FDG-avid nodule with peripheral edema in the right lateral ventricle, measuring approximately 1 cm in diameter with a SUVmax of 4.41, suggesting metastatic disease (Figure 1C). Contrast-enhanced magnetic resonance imaging (MRI) of the head indicated multiple intracranial metastasis. Pathological tissue obtained via lung puncture confirmed the presence of NSCLC. Through consultation with another local tertiary A-grade hospital, we identified the tumor as NSCLC not otherwise specified (NSCLC-NOS). The results of the immunohistochemical staining were as follows: cytokeratin (pan) (+), cytokeratin 7 (focal+), P40 (−), P63 (−), TTF-1 (−), napsin A (−), synaptophysin (partial+), CD56 (−), Ki-67 (70%+), NUT (−), SMARCA4 (−), and PD-L1 tumor proportion score (TPS) <1% (Figure 2A,2B). Next-generation sequencing (NGS) was performed at Yunying Medical Inspection Institute, and the NGS report was provided by the patient himself. Genetic testing was performed on the patient, and detection of single-nucleotide variants and insertion-deletion (SNV/InDel) mutations, including the following genes were analyzed: AKT1, ALK, BIM, BRAF, DDR2, EGFR, ERBB2, FGFR1, FGFR3, HRAS, KRAS, MAP2K1, MET, NRAS, NTRK1, NTRK2, NTRK3, PDGFRA, PIK3CA, PTEN, RET, ROS1, TP53. Results revealed a TP53 exon 10 c.1015G>T (p.E339*) mutation with a mutation abundance of 46.5%. Gene fusion testing detected no gene fusion mutations. Copy number variation (CNV) analysis showed no copy number mutations. Tumor mutation burden (TMB) was not tested. Overall analysis: the patient is male with a history of smoking and presented with multiple metastases at the time of diagnosis. Histopathological morphology indicated undifferentiated features (distinguishing it from patients with lung cancer without SMARCA4 protein expression, which typically retain histological features of adenocarcinoma or squamous cell carcinoma differentiation). Immunohistochemistry (IHC) demonstrated loss of SMARCA4, P40 negativity, TTF-1 negativity, and P63 negativity. Comprehensive analysis supports the diagnosis of SMARCA4-UT.


The patient received a combination of camrelizumab [200 mg, every 3 weeks (Q3W)], bevacizumab (7.5 mg/kg, Q3W), pemetrexed (500 mg/m2, Q3W), and carboplatin (area under the curve 5, Q3W) beginning on January 27, 2022 with remarkable clinical improvement. This regimen was continued for four cycles, followed by camrelizumab (200 mg, Q3W), bevacizumab (7.5 mg/kg, Q3W), and pemetrexed (500 mg/m2, Q3W) maintenance therapy for 31 cycles. The final treatment was on June 25, 2024 (Figure 3). CT (Figure 4) and MRI (Figure 5) scans indicated that the best response was partial reduction. According to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, the tumor mass decreased over 30% from the pretreatment size (11). The patient’s only adverse reaction was mild hypertension (grade 2). At the time of writing, the patient is still benefiting from the above regimen after 32 months of treatment, with the most recent CT scan showing a tumor reduction of −52% compared with baseline.


All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from relatives of the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International multidisciplinary team (iMDT) discussion
Principal findings
We treated the patient with the combination of camrelizumab, bevacizumab, pemetrexed, and carboplatin. To our knowledge, this is the first documented case of SMARCA4-UT treated with this combined regimen, which achieved a progression-free survival (PFS) of 32 months. This therapy was well tolerated and led to long-term response.
Literature comparison
Naito et al. (12) reported a case of a 43-year-old male patient diagnosed with SMARCA4-deficient lung adenocarcinoma who was treated with nivolumab after failing in three standard chemotherapy protocols. The best response was partial response with a PFS of over 14 months. Lin et al. (13) compared the efficacy of traditional chemotherapy and immune checkpoint inhibitors (ICIs) plus chemotherapy in advanced thoracic tumors with SMARCA4-deletion, the results of which revealed comparable response rates. However, the median PFS of the former was significantly shorter than that of the latter when applied as first-line treatment (2.73 vs. 26.8 months, P=0.044), and patients with ICIs in first-line had a significantly longer overall survival than without ICIs or in later lines. This suggests that immunotherapy may be an effective therapeutic option for patients with SMARCA4-UTs and that combination with chemotherapy may enhance the treatment effect.
Additionally, the combination of anti-programmed death-1 (PD-1)/PD-L1 antibodies and antiangiogenic agents may exert a synergistic effect (14). Kawachi et al. (10) reported that three patients with SMARCA4-DTS who were treated with ABCP all achieved partial responses, with a PFS of approximately 6 months or longer and a continuous response of 1 year or longer in one case. Their report suggests that the ABCP regimen can be an effective treatment for those with SMARCA4-DTS. Ye et al. (15) reported the first known case of SMARCA4-deficient NSCLC to be treated with the combination of anlotinib, nab-paclitaxel, and tislelizumab, which led to partial response and a PFS of 5 months. In contrast, our patient received a classical platinum doublet combined with camrelizumab and bevacizumab resulting in an approximately six-fold longer survival. We are currently planning a prospective study in this population to further validate the efficacy and safety of this therapy.
Interpretation of findings
SMARCA4 is a tumor-suppressor gene encoding the SMARCA4/BRG1 protein on chromosome 19p13.2 (16), which is one of the important subunits of the SWI-SNF complex, and SMARCA4 mutations may cause loss of expression of the SMARCA4/BRG1 protein. The SWI-SNF complex is an evolutionarily highly conserved ATP-dependent chromatin remodeling complex, which is the earliest discovered and well-known chromatin remodeling complex, and plays an important role in the regulation of important cellular processes and functions, such as gene transcription, proliferation, differentiation, and DNA repair (17). Research has demonstrated that the SWI-SNF complex, which is composed of at least 15 protein subunits encoded by nearly 22 genes, exerts a critical tumor-suppressor effect (18). However, its core element consists of ATPase-dependent catalytic subunits, including SMARCA2 and SMARCA4. Mutations in SMARCA4 occur in 5% to 7% of all malignancies, including lung cancer, colon cancer, bladder cancer, breast cancer, small cell carcinoma of the ovary of hypercalcemic type, thoracic sarcoma, and uterine sarcoma (19-21). Nevertheless, this does not necessarily imply a link between SMARCA4 gene mutations and a loss of SMARCA4 expression. Rekhtman et al. (1) found that the IHC results of two patients with poorly differentiated thoracic malignant tumors indicated a loss of SMARCA4 expression, but the corresponding gene deletion mutation was not found in second-generation gene sequencing. The same phenomenon was observed in our case. Most of the related research suggests the SMARCA4 gene mutation is clinically significant and the value of SMARCA4 deficiency based on IHC analysis may also have important ramifications. Future studies with larger sample sizes are needed to determine whether a definitive diagnosis should be based on the absence of immunohistochemical expression or a genetic mutation.
As opposed to EGFR or ALK/ROS1 mutated tumors, there is currently no targeted drug at hand for SMARCA4-deficient thoracic tumors. According to preclinical data, CDK4/6 (22), AURKA (23), ATR (24), and TopoII (25) inhibitors can exert antitumor activity. Further clinical trials based on these agents are ongoing.
Vascular endothelial growth factor (VEGF)-mediated immunosuppression in the tumor microenvironment (TME) and its negative influence on the efficacy of immunotherapy constitute the theoretical basis for the combination of PD-1/PD-L1 antibodies and anti-VEGF drugs, which may normalize TME (26). Traditional cytotoxic chemotherapy drugs stimulate antitumor immunity through the release of tumor-associated antigens and/or the depletion of immunosuppressive cells (27,28).
Implications and future direction
Certain limitations regarding the conclusion drawn from this case should be noted. As we only report a single case, further prospective multicenter randomized controlled trials with large sample sizes are needed to confirm the efficacy and safety of this therapy. Moreover, it is also necessary to further explore the molecular biological mechanism in basic experiments and functional experiments to clarify the potential mechanisms. Accurately identifying SMARCA4-UT and providing more precise and effective treatment methods to improve the quality of life and survival rate of patients remain clinical challenges that need to be addressed.
Discussion of the Thoracic Cancer Physicians at Liaoning Cancer Hospital & Institute
In this case, the first-line combination therapy consisting of immunotherapy combined with anti-angiogenic therapy and chemotherapy has demonstrated durable efficacy for the patient, which may provide a novel therapeutic option for this patient population. Regarding subsequent treatment strategies, several issues warrant further exploration.
Selection of maintenance therapy: the patient is currently undergoing maintenance therapy with camrelizumab, bevacizumab, and pemetrexed, with a PFS exceeding 32 months. De-escalation of maintenance therapy may be considered. The choice of maintenance regimen should integrate assessments of adverse reactions, imaging findings, tumor marker levels, and minimal residual disease monitoring to evaluate tumor burden dynamics and guide adjustments in treatment strategies.
Combination therapy modalities: ICIs combined with chemotherapy or anti-angiogenic agents have shown significant clinical benefits. Concurrently, the synergistic application of immunotherapy and radiotherapy has demonstrated remarkable efficacy, offering new insights for optimizing therapeutic approaches. However, high-quality evidence-based medical evidence remains lacking in this area.
Discussion between oncologists from The Christie NHS Foundation Trust and Paracelsus Medical University Salzburg University Hospital on issues related to the diagnosis and treatment of this patient:
Question 1: What are the current first-line treatment options for patients with advanced SMARCA4-UT?
Expert opinion 1: Dr. Franz Zehentmayr
When patients present with resectable tumors and in good physical shape, the therapeutic mainstay is radical surgery. However, if the tumor is regarded as inoperable, which is mostly the case as patients with this rare disease entity usually present in advanced stages, thoracic radiotherapy as a loco-regional treatment option can be applied despite these SMARCA4-deficient undifferentiated tumors are said to be insensitive to ionizing radiation (29). Irradiation treatment can be combined with chemotherapy both to enhance the effect of loco-regional therapy and to account for the systemic disease spread. In recent years, immune checkpoint inhibition has come into play, as a very effective treatment option for non-resectable as well as for resectable lung cancers so that it seems—based on these data—that immunotherapy combined with either form of local therapy, i.e., surgery or radiotherapy, paves the way for additional treatment options (30-35). Furthermore, in analogy to epitheloid sarcomas with loss of SMARCB1, tazemetostat—which was successfully tested in a phase 2 trial—could serve as a specific targeted agent also in patients with SMARCA4 (36). This approach, however, has not been investigated thus far.
Expert opinion 2: Dr. Igor Gomez-Randulfe
Advanced SMARCA4-UTs lack a universally accepted standard of care due to their rarity and aggressive behavior. Traditional cytotoxic chemotherapy has shown limited efficacy and poor outcomes. However, emerging evidence from case reports and small series indicates that integrating ICIs into first-line regimens may improve responses. Publishing cases like the one presented is crucial for understanding the efficacy of these combination strategies in this rare tumor.
Question 2: What is the significance of IHC and genetic testing for the pathologic diagnosis of SMARCA4?
Expert opinion 1: Dr. Franz Zehentmayr
IHC and molecular testing are prerequisites for exact pathologic diagnosis since these tumors are undifferentiated high-grade neoplasms that are hard to characterize. They usually show homozygosity with a loss of BRG1 that seems to be crucial for tumorigenesis (37). High mutational burden with concurrent mutations e.g., in KRAS may make these patients liable to good responses to immunotherapy even if they are PD-L1 negative (37-39). Hence, in light of the above-mentioned potential benefits of immunotherapy and targeted therapies in this disease entity, IHC and extensive molecular testing are indispensable to provide a basis adequate treatment selection.
Expert opinion 2: Dr. Igor Gomez-Randulfe
IHC plays an essential role in the pathologic diagnosis of SMARCA4-deficient tumors by directly demonstrating the loss of SMARCA4/BRG1 protein expression. This loss, as highlighted in the case report through negative SMARCA4 staining, is critical for the diagnosis of these aggressive tumors. IHC is both a rapid and cost-effective tool, enabling pathologists to distinguish SMARCA4-UT from other thoracic malignancies, which often have overlapping morphologic features.
Genetic testing, including NGS, complements IHC by detecting underlying mutations or structural alterations in the SMARCA4 gene. Interestingly, there can be discordance between IHC and genetic findings; for instance, loss of protein expression may be present even in the absence of a detectable gene mutation, possibly due to other genetic structural variations. Therefore, the combination of IHC and molecular genetic testing provides a more comprehensive diagnostic approach, guiding not only the accurate classification of the tumor but also informing potential therapeutic strategies and prognosis.
Question 3: What are the suggestions for this case report?
Expert opinion 1: Dr. Franz Zehentmayr
The diagnostic panel including IHC and molecular testing for thoracic tumors which, as said before, is indispensable for exact diagnosis and the basis for adequate therapy also proved useful in this 71-year-old patient. With 32 months, this patient is on the upper edge of known survival rates for SMARCA4-deficient undifferentiated tumors. The treatment regimen including camrelizumab combined with bevacizumab and platinum-based chemotherapy is an unusual but, in this case, very successful therapeutic concept, which merits further investigation in larger prospective studies. Even more so, since camrelizumab has shown efficacy in a phase III trial in non-squamous cell carcinomas of the lung (40). By means of successful systemic treatment loco-regional approaches such as stereotactic radiotherapy for brain or lung lesions can be saved for a later timepoint when the patient might present with localized tumor progression. Individualized approaches like the one presented in the current case help sparing treatment-related toxicity in an increasingly elderly and frail patient population.
Expert opinion 2: Dr. Igor Gomez-Randulfe
This case report offers important insights into a rare disease with limited available evidence. Given the low response rate of chemotherapy alone, the use of a quadruple regimen combining an ICI, an antiangiogenic agent, and chemotherapy is a rational strategy supported by biological plausibility. The patient’s sustained benefit highlights the potential of this approach and lays the groundwork for further research. Recognizing that prospective trials may be challenging in this setting, the authors are encouraged to consider establishing an international registry to collect data on various treatment regimens. Such an initiative would enhance our collective understanding and ultimately guide optimal management strategies for this challenging malignancy.
Conclusions
To our knowledge, this is the first report of a patient with SMARCA4-UT treated with a combined first-line regimen of immunotherapy plus antiangiogenic therapy and chemotherapy. Treatment resulted in durable response with acceptable toxicity Although this regimen shows promise, further prospective studies are needed to validate its efficacy and safety.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-541/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-541/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-541/coif). I.G.R. reports that he receives consulting fees, and support for attending meetings and travel from Janssen, and participates on Advisory Board of Janssen. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from relatives of the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J Thorac Oncol 2020;15:231-47. [Crossref] [PubMed]
- Gandhi JS, Alnoor F, Sadiq Q, et al. SMARCA4 (BRG1) and SMARCB1 (INI1) expression in TTF-1 negative neuroendocrine carcinomas including merkel cell carcinoma. Pathol Res Pract 2021;219:153341. [Crossref] [PubMed]
- La Fleur L, Falk-Sörqvist E, Smeds P, et al. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Cancer 2019;130:50-8. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Zhou P, Fu Y, Tang Y, et al. Thoracic SMARCA4-deficient undifferentiated tumor: A clinicopathological and prognostic analysis of 35 cases and immunotherapy efficacy. Lung Cancer 2024;189:107471. [Crossref] [PubMed]
- Longo V, Catino A, Montrone M, et al. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. Int J Mol Sci 2024;25:3237. [Crossref] [PubMed]
- Li B, Li XG. Iodine-125 Seed Implantation and Transarterial Chemoinfusion Combined with Immune Checkpoint Inhibitors for a Thoracic SMARCA4-Deficient Undifferentiated Tumor. J Vasc Interv Radiol 2023;34:147-9. [Crossref] [PubMed]
- Gantzer J, Davidson G, Vokshi B, et al. Immune-Desert Tumor Microenvironment in Thoracic SMARCA4-Deficient Undifferentiated Tumors with Limited Efficacy of Immune Checkpoint Inhibitors. Oncologist 2022;27:501-11. [Crossref] [PubMed]
- Shi L, Lin L, Ding Y, et al. Case report: A rapid response to immunotherapy in a thoracic SMARCA4-deficient undifferentiated tumor with respiratory failure. Front Oncol 2022;12:1020875. [Crossref] [PubMed]
- Kawachi H, Kunimasa K, Kukita Y, et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy 2021;13:799-806. [Crossref] [PubMed]
- Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol 2019;30:1401-3. [Crossref] [PubMed]
- Naito T, Umemura S, Nakamura H, et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thorac Cancer 2019;10:1285-8. [Crossref] [PubMed]
- Lin Y, Yu B, Sun H, et al. Promising efficacy of immune checkpoint inhibitor plus chemotherapy for thoracic SMARCA4-deficient undifferentiated tumor. J Cancer Res Clin Oncol 2023;149:8663-71. [Crossref] [PubMed]
- Hanna NH, Temin S, Masters G. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update Summary. JCO Oncol Pract 2020;16:e844-8. [Crossref] [PubMed]
- Ye R, Wu A, Lin C, et al. SMARCA4-deficient non-small cell lung cancer: a case description and literature analysis. Quant Imaging Med Surg 2024;14:4215-22. [Crossref] [PubMed]
- Rodriguez-Nieto S, Sanchez-Cespedes M. BRG1 and LKB1: tales of two tumor suppressor genes on chromosome 19p and lung cancer. Carcinogenesis 2009;30:547-54. [Crossref] [PubMed]
- Mardinian K, Adashek JJ, Botta GP, et al. SMARCA4: Implications of an Altered Chromatin-Remodeling Gene for Cancer Development and Therapy. Mol Cancer Ther 2021;20:2341-51. [Crossref] [PubMed]
- Li Y, Yang X, Zhu W, et al. SWI/SNF complex gene variations are associated with a higher tumor mutational burden and a better response to immune checkpoint inhibitor treatment: a pan-cancer analysis of next-generation sequencing data corresponding to 4591 cases. Cancer Cell Int 2022;22:347. [Crossref] [PubMed]
- AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov 2017;7:818-31. [Crossref] [PubMed]
- Peng L, Li J, Wu J, et al. A Pan-Cancer Analysis of SMARCA4 Alterations in Human Cancers. Front Immunol 2021;12:762598. [Crossref] [PubMed]
- Chetty R, Serra S. SMARCA family of genes. J Clin Pathol 2020;73:257-60. [Crossref] [PubMed]
- Xue Y, Meehan B, Fu Z, et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun 2019;10:557. [Crossref] [PubMed]
- Tagal V, Wei S, Zhang W, et al. SMARCA4-inactivating mutations increase sensitivity to Aurora kinase A inhibitor VX-680 in non-small cell lung cancers. Nat Commun 2017;8:14098. [Crossref] [PubMed]
- Gupta M, Concepcion CP, Fahey CG, et al. BRG1 Loss Predisposes Lung Cancers to Replicative Stress and ATR Dependency. Cancer Res 2020;80:3841-54. [Crossref] [PubMed]
- Fillmore CM, Xu C, Desai PT, et al. EZH2 inhibition sensitizes BRG1 and EGFR mutant lung tumours to TopoII inhibitors. Nature 2015;520:239-42. [Crossref] [PubMed]
- Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol 2020;11:598877. [Crossref] [PubMed]
- Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer 2020;2:zcaa002. [Crossref] [PubMed]
- Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35. [Crossref] [PubMed]
- Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient Thoracic Sarcomas: Clinicopathologic Study of 30 Cases With an Emphasis on Their Nosology and Differential Diagnoses. Am J Surg Pathol 2019;43:455-65. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Horita N, Fujiwara Y. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 2024;390:287. [Crossref] [PubMed]
- Forde PM, Spicer J, Girard N. Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer. N Engl J Med 2022;387:572-3. Reply. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Gounder M, Schöffski P, Jones RL, et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol 2020;21:1423-32. [Crossref] [PubMed]
- Nambirajan A, Jain D. Recent updates in thoracic SMARCA4-deficient undifferentiated tumor. Semin Diagn Pathol 2021;38:83-9. [Crossref] [PubMed]
- Tan AC, Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol 2022;40:611-25. [Crossref] [PubMed]
- Ruznic E, Klebermass M, Zellinger B, et al. Immunotherapy Improves Clinical Outcome in Kirsten Rat Sarcoma Virus-Mutated Patients with Unresectable Non-Small Cell Lung Cancer Stage III: A Subcohort Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR). J Clin Med 2025;14:945. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
(English Language Editor: J. Gray)


