
Association between reactive cutaneous capillary endothelial proliferation and the efficacy of camrelizumab in esophageal cancer: a retrospective cohort study
Highlight box
Key findings
• The occurrence of reactive cutaneous capillary endothelial proliferation (RCCEP) is closely associated with the efficacy of camrelizumab in the treatment of esophageal cancer (EC), specifically in terms of the objective response rate, disease control rate, progression-free survival (PFS), and overall survival (OS).
What is known and what is new?
• RCCEP is one of the most common immune-related adverse events (irAEs) of camrelizumab. The occurrence of RCCEP is positively correlated with the prognosis of non-small cell lung cancer and advanced hepatocellular carcinoma.
• In this study, the patients with RCCEP had better outcomes than those with other irAEs. Moreover, RCCEP time and grade had no relationship with extended PFS nor OS.
What is the implication, and what should change now?
• RCCEP was found to be a predictor of camrelizumab efficacy in the treatment of EC; thus, large prospective trials need to be conducted to confirm these findings. In addition, the mechanism of RCCEP was found to be able to predict the efficacy of EC treatments, which also needs to be further explored in future studies.
Introduction
Esophageal cancer (EC) is the seventh most common cancer and the sixth leading cause of cancer-related death worldwide (1). Traditional treatments, including chemotherapy and radiotherapy, have limited efficacy, and can cause serious adverse events. The emergence of immune checkpoint inhibitors (ICIs) has fostered renewed optimism in tumor treatment, and ICIs have been widely used to treat tumors such as EC, non-small cell lung cancer (NSCLC), melanoma, and renal cell carcinoma (1,2). ICIs relieve T-cell inhibition, and reactivate their anti-tumor cytolytic function by blocking the inhibitory pathway between T lymphocytes and tumor cells or antigen-presenting cells, thereby enhancing anti-tumor immunity (3). The two major classes of ICIs currently used in cancer immunotherapy are monoclonal antibodies targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4), and the programmed cell death 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) axis. Compared with cytotoxic chemotherapy alone, the combination of chemotherapy with several ICIs, including nivolumab, pembrolizumab, and camrelizumab, has been shown to extend the overall survival (OS) and progression-free survival (PFS) of EC patients (4-6). However, ICIs not only boost the immune system of patients to fight disease but also enhance their body’s immune response, resulting in immune-related adverse events (irAEs) (1). Unlike adverse events mediated by traditional cytotoxic drugs, irAEs are associated with an immune-mediated mechanism of action and may impact any other organ systems in the body. The most frequently affected organs include the skin, endocrine system, heart, gastrointestinal (GI) system, lungs, and liver (7,8). Severe irAEs can result in the discontinuation of patients’ ICI anti-tumor treatment and can even be life-threatening (9,10). Since irAEs arise from a process of immune activation, this suggests that exhausted immune cells have been reinvigorated and are capable of attacking not only tumor cells but also normal tissues. Theoretically, the occurrence of irAEs may suggest a better response to ICI therapy. However, it remains unclear whether the occurrence of irAEs is associated with improved ICI outcomes in patients.
Camrelizumab, an ICI that targets PD-1, was developed by a Chinese pharmaceutical company. Based on the results of the Esophageal Squamous Cell Carcinoma Immunotherapy Research Trial (ESCORT)-1st trial, on December 8, 2021, camrelizumab was approved by the China National Medical Products Administration as the first-line treatment for patients with unresectable locally advanced/relapsed or metastatic EC in combination with paclitaxel and cisplatin (11). Other clinical studies have shown that camrelizumab combined with chemotherapy can be used as a neoadjuvant therapy for locally advanced EC (12-14). Camrelizumab is a humanized, high-affinity immunoglobulin G4 kappa monoclonal antibody against PD-1. It can activate CD4+ T cells and increase the release of interleukin (IL)-4. IL-4 is an inflammatory factor produced by T helper 2 (Th2) cells. IL-4 stimulates the differentiation of CD163+ M2 macrophages, which promotes vascular regeneration by releasing vascular endothelial growth factor (VEGF)-A (15). In addition, camrelizumab acts on cells expressing PD-1 in the skin, which may lead to the synthesis of VEGF through the release of chemokines (16). It is thought that the enhanced immune response induced by camrelizumab may disrupt the dynamic balance between angiogenic and anti-angiogenic factors, leading to reactive cutaneous capillary endothelial proliferation (RCCEP). So far, the exact mechanisms of RCCEP remain unknown. RCCEP was the most frequently observed immune-related adverse reaction associated with camrelizumab treatment (11), and originally thought to be an irAE specific to camrelizumab. However, recent observations have indicated that RCCEP also occurs in patients with metastatic melanoma who are treated with nivolumab or pembrolizumab, although the incidence rate is relatively low at 2.4% (17). Previous studies have reported a positive correlation between RCCEP occurrence and prognosis in patients with NSCLC or advanced hepatocellular carcinoma (HCC) treated with carrilizumab (9,18). However, it remains unclear whether RCCEP occurrence can predict the efficacy of camrelizumab in treating patients with EC.
The strict eligibility criteria of randomized controlled trials may limit their generalizability to daily clinical practice and cannot fully capture the complexities of real-world treatment scenarios, we decided to conduct a real-world study. This study was conducted to investigate the association between RCCEP and the outcomes of camrelizumab-treated EC patients and to identify any related factors. Additionally, this study sought to clarify the risk factors for RCCEP to identify patients at high risk of RCCEP and to provide a foundation for future research on its mechanism of occurrence. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-366/rc).
Methods
Study design and patients
This was a single-center retrospective cohort study of EC patients who were treated with camrelizumab at The Fourth Hospital of Hebei Medical University between November 2019 and November 2023. The last follow-up date was on February 2, 2024. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) had histologically confirmed EC; (II) had received at least one cycle of camrelizumab monotherapy or combined standard therapy (chemoradiotherapy or targeted therapy); (III) aged ≥18 years; (IV) had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–3; (V) had measurable lesions according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); and (VI) had complete imaging data obtained before and after treatment. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had previously received an immunotherapy treatment before the administration of camrelizumab; (II) had a history of autoimmune disease; (III) had a history of malignant tumors or other malignant tumors; (IV) had received neoadjuvant therapy with camrelizumab and had undergone surgical resection of EC after treatment; (V) had uncontrolled organic disease or infection, such as decompensated heart failure, lung failure, or kidney failure; and/or (VI) had incomplete or missing medical records. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethical Research Committee of The Fourth Hospital of Hebei Medical University (approval No. 2022KS014). Due to the retrospective nature of the study, the requirement of informed consent was waived.
Data collection and outcomes
Before initiating camrelizumab treatment, the medical records and the following patient characteristics were reviewed: patient age, sex, laboratory data, ECOG PS score, family history of cancer, stage, histology, metastatic status, history of alcohol consumption, history of smoking, lactate dehydrogenase (LDH) level, number of camrelizumab treatment cycles completed, combination treatment regimen (chemoradiotherapy, or targeted therapy), immunotherapy line, the start and end dates of camrelizumab treatment, the date of clinical or radiological disease progression based on RECIST 1.1 criteria, the date of death, the last known follow-up date (if the date of death was not recorded), as well as the grade and onset date of irAEs. Data regarding irAEs and laboratory examination values were collected from clinical notes, including hospital records. All irAEs were graded by senior physicians based on the Common Terminology Criteria for Adverse Events version 5.0. The grading criteria for RCCEP were defined as follows: grade 1: nodule(s) with a maximum diameter of ≤10 mm, with or without rupture and bleeding; grade 2: nodule(s) with a maximum diameter of >10 mm, with or without rupture and bleeding; grade 3: generalized nodules throughout the body, potentially complicated by skin infections; grade 4: multiple and generalized nodules accompanied by a life-threatening condition; and grade 5: death. We defined the timing of RCCEP as the interval from the first camrelizumab dose to the occurrence of RCCEP. OS was calculated as the time from the first camrelizumab dose to death due to any causes. PFS was calculated as the time from the first camrelizumab dose to disease progression (clinical and/or radiographic) or death from any causes. The RECIST 1.1 was used to assess the treatment response. The objective response rate (ORR) was defined as the percentage of patients who achieved a complete response (CR) or partial response (PR) to immunotherapy. The disease control rate (DCR) was defined as the percentage of patients who had CR, PR, or stable disease (SD).
Prognostic nutritional index (PNI) was calculated by the formula 10 × albumin (g/dL) + 0.005 × lymphocyte count (/µL) (19). Body mass index (BMI) was defined as the weight before treatment divided by the square of the height; that is: BMI = weight (kg)/height (m2). In accordance with previous research results (20-23), the truncation value of albumin, BMI, PNI, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) were set at 36.8 g/L, 18.5 kg/m2, 45.7, 5, and 152, respectively.
The patients were divided into an RCCEP-positive group (with RCCEP or RCCEP+) and an RCCEP-negative group (without RCCEP or RCCEP−) based on the occurrence of RCCEP. Additionally, based on the occurrence of both RCCEP and irAEs, patients were categorized into a group that was RCCEP positive (with RCCEP or RCCEP+), a group that was irAE positive but RCCEP negative (with irAEs but without RCCEP or irAEs+, RCCEP−), and a group that was irAE negative (without irAEs or irAEs−). Differences in efficacy were analyzed according to the occurrence of RCCPE and irAEs.
Statistical analysis
The categorical data were described as the number (percentage), and the quantitative data were described as the median (range). The Chi-squared test was used to analyze the categorical data, and the Student’s t-test was used to analyze the quantitative data. The survival data were evaluated by both Kaplan-Meier analysis and the log-rank test. Univariate and multivariate Cox regression analyses were conducted to identify potential predictive factors for PFS and OS. Variables with P value ≤0.10 in univariate analyses were included in the multivariate model, statistical significance was defined as P<0.05. The predictive factors of RCCEP were analyzed by logistic regression analyses. A two-tailed P value <0.05 was considered statistically significant. For the patients with RCCEP, as the timing of RCCEP onset varied widely, we incorporated RCCEP latency as a time-dependent covariate in the regression models. To identify a specific cut-off time when RCCEP development was most likely to influence outcomes, RCCEP latency was transformed from a continuous covariate to an ordinal variable with 1-month intervals in a time-dependent Cox regression model. To ensure that early death in patients with RCCEP did not interfere in determining the relationship between RCCEP timing and efficacy, we conducted a 6-month landmark analysis of the patients who developed RCCEP within 6 months after receiving camrelizumab treatment but who remained alive after this landmark time point. We used SPSS 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) for the statistical analyses and data visualization.
Results
Patient characteristics
In total, 397 patients were included in the study. The patients had a median age of 66 (range, 27–87) years, and the majority were male (n=291, 73.30%). Their demographics and clinical backgrounds are detailed in Table 1. There were no significant differences in the baseline characteristics between the patients with and without RCCEP (Table 1).
Table 1
| Characteristics | All patients | RCCEP− group | RCCEP+ group | P value |
|---|---|---|---|---|
| Total | 397 (100.00) | 269 (67.76) | 128 (32.24) | – |
| Age (years) | 66 [27–87] | 66 [27–87] | 66 [42–84] | 0.18 |
| Sex | 0.16 | |||
| Female | 106 (26.70) | 66 (16.62) | 40 (10.08) | |
| Male | 291 (73.30) | 203 (51.13) | 88 (22.17) | |
| ECOG PS | 0.55 | |||
| <2 | 371 (93.45) | 250 (62.97) | 121 (30.48) | |
| ≥2 | 26 (6.55) | 19 (4.79) | 7 (1.76) | |
| Family history of cancer | 0.16 | |||
| No | 285 (71.79) | 199 (50.13) | 86 (21.66) | |
| Yes | 112 (28.21) | 70 (17.63) | 42 (10.58) | |
| Histology | 0.76 | |||
| Squamous cell carcinoma | 385 (96.98) | 260 (65.49) | 125 (31.49) | |
| Other | 12 (3.02) | 9 (2.27) | 3 (0.76) | |
| Metastasis | 0.67 | |||
| No | 211 (53.15) | 141 (35.52) | 70 (17.63) | |
| Yes | 186 (46.85) | 128 (32.24) | 58 (14.61) | |
| Drinking history | 0.92 | |||
| No | 200 (50.38) | 136 (34.26) | 64 (16.12) | |
| Yes | 197 (49.62) | 133 (33.50) | 64 (16.12) | |
| Smoking history | 0.07 | |||
| No | 188 (47.36) | 119 (29.97) | 69 (17.38) | |
| Yes | 209 (52.64) | 150 (37.78) | 59 (14.86) | |
| LDH level | 0.11 | |||
| ≤ ULN | 380 (95.72) | 254 (63.98) | 126 (31.74) | |
| > ULN | 17 (4.28) | 15 (3.78) | 2 (0.50) | |
| Stage | 0.46 | |||
| I | 9 (2.27) | 5 (1.26) | 4 (1.01) | |
| II | 75 (18.89) | 49 (12.34) | 26 (6.55) | |
| III | 162 (40.81) | 106 (26.70) | 56 (14.11) | |
| IV | 151 (38.04) | 109 (27.46) | 42 (10.58) | |
| Primary EC | 0.09 | |||
| Upper thoracic | 105 (26.45) | 68 (17.13) | 37 (9.32) | |
| Middle thoracic | 197 (49.62) | 128 (32.24) | 69 (17.38) | |
| Lower thoracic | 95 (23.93) | 73 (18.39) | 22 (5.54) |
Data are presented as n (%) or median [range]. EC, esophageal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; RCCEP, reactive cutaneous capillary endothelial proliferation; ULN, upper limit of normal.
Treatment patterns
Of the 397 patients, 297 (74.81%) received camrelizumab in the 1st line, 94 (23.68%) in the 2nd line, and 6 patients (1.51%) in the third line and above. Of the patients, 52 received immunotherapy alone, 320 received immunotherapy combined with chemotherapy, and 25 received immunotherapy combined with anti-angiogenic therapy. The median cycle was 3 (range, 1–38) times, and the median duration of camrelizumab treatment was 106 (range, 21–1,137) days. The specific treatment patterns are shown in Table 2. Of the patients treated with camrelizumab, 71 received one cycle of camrelizumab, 69 received two cycles, and 59 received three cycles. The number of patients by treatment cycles are shown in Figure 1.
Table 2
| Variables | Data (n=397) |
|---|---|
| Treatment line, n (%) | |
| 1 | 297 (74.81) |
| 2 | 94 (23.68) |
| ≥3 | 6 (1.51) |
| Systemic treatment, n (%) | |
| Camrelizumab alone | 52 (13.10) |
| Camrelizumab plus chemotherapy | 320 (80.60) |
| Albumin-bound paclitaxel plus platinum | 179 (45.09) |
| Paclitaxel plus platinum | 43 (10.83) |
| Albumin-bound paclitaxel | 19 (4.79) |
| Docetaxel plus platinum | 19 (4.79) |
| Paclitaxel | 12 (3.02) |
| S-1 (tegafur-gimeracil-oteracil potassium) | 9 (2.27) |
| Fluorouracil plus platinum plus folinic acid calcium salt hydrate | 8 (2.02) |
| Capecitabine | 6 (1.51) |
| Platinum | 5 (1.26) |
| Capecitabine plus platinum | 4 (1.01) |
| Etoposide plus platinum | 4 (1.01) |
| Fluorouracil plus platinum | 3 (0.76) |
| Irinotecan | 2 (0.50) |
| S-1 plus platinum | 2 (0.50) |
| Irinotecan plus capecitabine | 1 (0.25) |
| Fluorouracil plus irinotecan plus folinic acid calcium salt hydrate | 1 (0.25) |
| Albumin-bound paclitaxel plus S-1 | 1 (0.25) |
| Fluorouracil | 1 (0.25) |
| Fluorouracil plus folinic acid calcium salt hydrate | 1 (0.25) |
| Camrelizumab plus chemotherapy and antiangiogenic therapy | 10 (2.52) |
| Docetaxel plus platinum plus apatinib | 3 (0.76) |
| Paclitaxel plus platinum plus apatinib | 2 (0.50) |
| Albumin-bound paclitaxel plus apatinib | 2 (0.50) |
| Etoposide plus platinum plus apatinib | 1 (0.25) |
| Fluorouracil plus platinum plus folinic acid calcium salt hydrate plus apatinib | 1 (0.25) |
| Irinotecan plus S-1 plus apatinib | 1 (0.25) |
| Camrelizumab plus antiangiogenic therapy | 15 (3.78) |
| Apatinib | 8 (2.02) |
| Anlotinib | 5 (1.26) |
| Bevacizumab | 1 (0.25) |
| Recombinant human endostatin | 1 (0.25) |
| Combination of radiotherapy, n (%) | |
| No | 197 (49.62) |
| Yes | 200 (50.38) |
| Cycle of camrelizumab (times), median [range] | 3 [1–38] |
| Duration of camrelizumab (days), median [range] | 106 [21–1,137] |
Incidence of irAEs
In total, 192 (48.36%) patients experienced irAEs. Of these patients, 28 (7.05%) experienced grade 3 or higher irAEs, but no grade 5 irAEs were observed. In total, 152 (35.32%) and 40 (8.49%) patients had a single irAE and multiple irAEs, respectively. In total, 128 (32.24%) patients suffered from RCCEP. Of the patients with RCCEP, seven (1.76%) had grade 3 RCCEP, but no patient had grade 4 or 5 RCCEP. Among the patients who experienced irAEs, 25 (6.30%) were treated with glucocorticoids for serious events, and 23 (5.80%) required hormonal replacement therapy for endocrine-related issues; however, no deaths due to irAEs occurred. For further details about the irAEs, see Table 3.
Table 3
| Category | Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Any | 242 (60.96) | 128 (32.24) | 86 (21.66) | 25 (6.30) | 3 (0.76) |
| RCCEP | 128 (32.24) | 85 (21.41) | 36 (9.07) | 7 (1.76) | 0 (0.00) |
| irTAEs | 46 (11.59) | 22 (5.54) | 21 (5.29) | 3 (0.76) | 0 (0.00) |
| Skin rash/itch | 17 (4.28) | 8 (2.02) | 5 (1.26) | 3 (0.76) | 1 (0.25) |
| Pneumonitis | 13 (3.27) | 1 (0.25) | 8 (2.02) | 4 (1.01) | 0 (0.00) |
| Cardiovascular toxicities | 7 (1.76) | 1 (0.25) | 5 (1.26) | 1 (0.25) | 0 (0.00) |
| Diarrhea/colitis | 5 (1.26) | 2 (0.50) | 1 (0.25) | 2 (0.50) | 0 (0.00) |
| Infusion-related reactions | 5 (1.26) | 1 (0.25) | 3 (0.76) | 1 (0.25) | 0 (0.00) |
| Elevated transaminase | 4 (1.01) | 0 (0.00) | 2 (0.50) | 1 (0.25) | 1 (0.25) |
| Nephritis | 4 (1.01) | 1 (0.25) | 1 (0.25) | 1 (0.25) | 1 (0.25) |
| Arthritis | 4 (1.01) | 3 (0.76) | 1 (0.25) | 0 (0.00) | 0 (0.00) |
| Hyperglycemia | 3 (0.76) | 2 (0.50) | 1 (0.25) | 0 (0.00) | 0 (0.00) |
| Myositis | 3 (0.76) | 1 (0.25) | 1 (0.25) | 1 (0.25) | 0 (0.00) |
| Adrenal hypofunction | 2 (0.50) | 1 (0.25) | 0 (0.00) | 1 (0.25) | 0 (0.00) |
| Neurovirulence | 1 (0.25) | 0 (0.00) | 1 (0.25) | 0 (0.00) | 0 (0.00) |
Data are presented as n (%). CTCAE-V 5.0, Common Terminology Criteria for Adverse Events Version 5.0; irTAEs, immune-related thyroid adverse events; RCCEP, reactive cutaneous capillary endothelial proliferation.
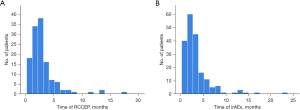
We also analyzed the incidence of irAEs associated with camrelizumab by cycle. We found that 82.81% (106/128) of RCCEP events occurred within 3 months of the first administration of camrelizumab, and the highest incidence of RCCEP was observed at 2–3 months (Figure 2A). A total of 242 irAEs occurred in 191 patients, 78.53% (150/191) of RCCEP events occurred within 3 months after the first administration of camrelizumab, and the highest incidence of irAEs was observed at 1–2 months (Figure 2B). The median follow-up time was 18.23 (range, 0.23–50.87) months.
Univariate and multivariate Cox analyses of PFS and OS
The univariate Cox analysis revealed that the >3 treatment cycles, the presence of irAEs or RCCEP, a BMI ≥18.5 kg/m2, albumin >36.8 g/L, and a PNI ≥45.7 were significantly associated with longer PFS. An ECOG PS score ≥2, LDH > upper limit normal (ULN), the ≥ 2nd line therapy, a NLR >5, and a PLR >152 were associated with shorter PFS. The variables with a P value ≤0.10 in the univariate Cox analysis were included in the multivariate Cox analysis. The multivariate analysis showed that the presence of RCCEP and albumin >36.8 g/L were independent factors associated with longer PFS. The ≥ 2nd line therapy and a NLR >5 were independent factors associated with shorter PFS (Table 4).
Table 4
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) (vs. <65) | |||||
| ≥65 | 0.97 (0.74–1.28) | 0.86 | |||
| Sex (vs. female) | |||||
| Male | 1.18 (0.86–1.62) | 0.30 | |||
| ECOG PS (vs. <2) | |||||
| ≥2 | 2.81 (1.78–4.43) | <0.001* | |||
| Family history of cancer (vs. no) | |||||
| Yes | 1.03 (0.76–1.39) | 0.87 | |||
| Histology (vs. squamous cell carcinoma) | |||||
| Other | 1.25 (0.59–2.67) | 0.56 | |||
| Metastasis (vs. no) | |||||
| Yes | 1.28 (0.98–1.68) | 0.08 | |||
| Drinking history (vs. no) | |||||
| Yes | 0.98 (0.75–1.29) | 0.88 | |||
| Smoking history (vs. no) | |||||
| Yes | 1.03 (0.78–1.35) | 0.85 | |||
| LDH level (vs. ≤ ULN) | |||||
| > ULN | 2.43 (1.39–4.28) | 0.02* | |||
| Cycles (vs. ≤3) | |||||
| >3 | 0.48 (0.36–0.63) | <0.001* | |||
| Treatment type (vs. monotherapy) | |||||
| Combined therapy | 0.74 (0.51–1.07) | 0.11 | |||
| Radiation (vs. no) | |||||
| Yes | 1.15 (0.88–1.52) | 0.31 | |||
| Line of therapy (vs. 1st) | |||||
| ≥ 2nd | 1.65 (1.23–2.22) | 0.001* | 1.47 (1.06–2.03) | 0.02* | |
| irAEs (vs. irAEs−) | |||||
| irAEs+ | 0.25 (0.18–0.34) | <0.001* | |||
| RCCEP (vs. RCCEP−) | |||||
| RCCEP+ | 0.10 (0.06–0.16) | <0.001* | 0.14 (0.08–0.25) | <0.001* | |
| BMI (kg/m2) (vs. <18.5) | |||||
| ≥18.5 | 0.61 (0.42–0.88) | 0.01* | |||
| Albumin (g/L) (vs. ≤36.8) | |||||
| >36.8 | 0.39 (0.28–0.55) | <0.001* | 0.64 (0.43–0.95) | 0.03* | |
| PNI (vs. <45.7) | |||||
| ≥45.7 | 0.70 (0.53–0.93) | 0.01* | |||
| NLR (vs. ≤5) | |||||
| >5 | 1.76 (1.31–2.37) | <0.001* | 1.54 (1.09–2.17) | 0.02* | |
| PLR (vs. ≤152) | |||||
| >152 | 1.97 (1.44–2.70) | <0.001* | |||
*, represents P<0.05. BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; irAEs, immune-related adverse events; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; RCCEP, reactive cutaneous capillary endothelial proliferation; ULN, upper limit of normal.
The univariate Cox analysis indicated that the >3 treatment cycles, the presence of irAEs or RCCEP, a BMI ≥18.5 kg/m2, albumin >36.8 g/L, and a PNI ≥45.7 were significantly associated with longer OS. An ECOG PS score ≥2, LDH > ULN, the ≥ 2nd line therapy, a NLR >5, and a PLR >152 were associated with shorter OS. The variables with a P value ≤0.10 in the univariate Cox analysis were included in the multivariate Cox analysis. The multivariate analysis revealed that the presence of irAEs or RCCEP and albumin >36.8 g/L were independent factors for longer OS. An ECOG PS score ≥2, the ≥ 2nd line therapy, and a NLR >5 were independent factors associated with shorter OS (Table 5).
Table 5
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) (vs. <65) | |||||
| ≥65 | 1.03 (0.79–1.36) | 0.82 | |||
| Sex (vs. female) | |||||
| Male | 1.18 (0.86–1.61) | 0.31 | |||
| ECOG PS (vs. <2) | |||||
| ≥2 | 3.29 (2.08–5.20) | <0.001* | 1.92 (1.15–3.23) | 0.01* | |
| Family history of cancer (vs. no) | |||||
| Yes | 0.97 (0.72–1.31) | 0.84 | |||
| Histology (vs. squamous cell carcinoma) | |||||
| Other | 0.99 (0.47–2.12) | 0.99 | |||
| Metastasis (vs. no) | |||||
| Yes | 1.21 (0.92–1.59) | 0.17 | |||
| Drinking history (vs. no) | |||||
| Yes | 0.98 (0.75–1.29) | 0.88 | |||
| Smoking history (vs. no) | |||||
| Yes | 1.03 (0.78–1.35) | 0.85 | |||
| LDH level (vs. ≤ ULN) | |||||
| > ULN | 1.95 (1.11–3.4) | 0.02 | |||
| Cycles (vs. ≤3) | |||||
| >3 | 0.44 (0.33–0.59) | <0.001* | |||
| Treatment type (vs. monotherapy) | |||||
| Combined therapy | 0.74 (0.51–1.07) | 0.11 | |||
| Radiation (vs. no) | |||||
| Yes | 1.12 (0.85–1.48) | 0.41 | |||
| Line of therapy (vs. 1st) | |||||
| ≥ 2nd | 1.51 (1.13–2.03) | <0.01* | 1.41 (1.02–1.94) | 0.04* | |
| irAEs (vs. irAEs−) | |||||
| irAEs+ | 0.23 (0.17–0.31) | <0.001* | 0.67 (0.46–0.97) | <0.03* | |
| RCCEP (vs. RCCEP−) | |||||
| RCCEP+ | 0.09 (0.06–0.16) | <0.001* | 0.14 (0.08–0.26) | <0.001* | |
| BMI (kg/m2) (vs. <18.5) | |||||
| ≥18.5 | 0.59 (0.41–0.85) | 0.004 | |||
| Albumin (g/L) (vs. ≤36.8) | |||||
| >36.8 | 0.36 (0.25–0.51) | <0.001 | 0.59 (0.40–0.88) | <0.01* | |
| PNI (vs. <45.7) | |||||
| ≥45.7 | 0.70 (0.53–0.93) | 0.01 | |||
| NLR (vs. ≤5) | |||||
| >5 | 1.72 (1.28–2.31) | <0.001 | 1.55 (1.10–2.19) | <0.01* | |
| PLR (vs. ≤152) | |||||
| >152 | 2.02 (1.47–2.77) | <0.001 | |||
*, represents P<0.05. BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; irAEs, immune-related adverse events; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; RCCEP, reactive cutaneous capillary endothelial proliferation; ULN, upper limit of normal.
Age, sex, a family history of cancer, histology, metastasis, a history of alcohol consumption, a history of smoking, treatment type, radiation, and immune-related thyroid adverse events (irTAEs) were not found to be associated with PFS nor OS (Tables 4,5).
Association between RCCEP and the efficacy of camrelizumab
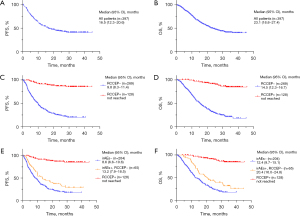
Compared to those without RCCEP, the patients with RCCEP had a higher ORR (71.09% vs. 43.87%, P<0.001) and DCR (94.53% vs. 72.49%, P<0.001) (Table 6). The 397 patients who received camrelizumab treatment had a median PFS of 16.5 months [95% confidence interval (CI): 12.3–20.6] (Figure 3A), and a median OS of 23.1 months (95% CI: 18.8–27.4) (Figure 3B). The patients in the RCCEP-positive group had a higher median PFS than those in the RCCEP-negative group [not reached vs. 9.8 months; hazard ratio (HR) =0.10; 95% CI: 0.06–0.16; P<0.001] (Figure 3C). Moreover, the patients in the RCCEP-positive group had a higher median OS than those in the RCCEP-negative group (not reached vs. 14.5 months; HR =0.09; 95% CI: 0.06–0.16; P<0.001) (Figure 3D). The patients in the RCCEP-positive group had a higher median PFS than those in the irAE-positive, RCCEP-negative group (not reached vs. 13.2 months, P<0.001); the patients in the RCCEP-positive group had a higher median PFS than those in the irAE negative group (not reached vs. 8.6 months, P<0.001); the patients in the irAE-positive, RCCEP-negative group had a higher median PFS than those in the irAE negative group (13.2 vs. 8.6 months, P=0.01) (Figure 3E). The patients in the RCCEP-positive group had a higher median OS than those in the irAE-positive, RCCEP-negative group (not reached vs. 20.4 months, P<0.001); the patients in the RCCEP-positive group had a higher median OS than those in the irAE-negative group (not reached vs. 12.4 months, P<0.001); the patients in the irAE-positive, RCCEP-negative group had a higher median OS than those in the irAE negative group (20.4 vs. 12.4 months, P=0.01) (Figure 3F).
Table 6
| Variables | All patients | RCCEP− group | RCCEP+ group | P value |
|---|---|---|---|---|
| Total, n | 397 | 269 | 128 | – |
| CR, n | 49 | 11 | 38 | – |
| PR, n | 160 | 107 | 53 | – |
| SD, n | 107 | 77 | 30 | – |
| PD, n | 81 | 74 | 7 | – |
| ORR (%) | 52.64 | 43.87 | 71.09 | <0.001 |
| DCR (%) | 79.60 | 72.49 | 94.53 | <0.001 |
CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; RCCEP, reactive cutaneous capillary endothelial proliferation; RECIST 1.1, Response Evaluation Criteria in Solid Tumor version 1.1; SD, stable disease.
In the time-dependent Cox regression model of patients who developed RCCEP and that included RCCEP latency as an ordinal variable, there was no difference in the PFS or OS between the patients who developed RCCEP 2–5 months and >5 months after the initiation of camrelizumab treatment compared to those who developed RCCEP within the first month of treatment (Table 7).
Table 7
| Variables | PFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Time to RCCEP | |||||
| ≤1 month | Reference | Reference | |||
| >1 and ≤2 months | 0.43 (0.06–3.06) | 0.40 | 0.37 (0.05–2.65) | 0.32 | |
| >2 and ≤3 months | 0.82 (0.15–4.46) | 0.82 | 0.69 (0.13–3.78) | 0.67 | |
| >3 and ≤5 months | 1.39 (0.27–7.19) | 0.69 | 1.21 (0.23–6.27) | 0.82 | |
| >5 months | 1.14 (0.16–8.10) | 0.90 | 0.95 (0.13–6.74) | 0.96 | |
| Grade of RCCEP | |||||
| 1 | Reference | Reference | |||
| 2 | 0.99 (0.30–3.21) | 0.98 | 0.91 (0.28–2.95) | 0.87 | |
| 3 | 2.69 (0.58–12.48) | 0.21 | 2.46 (0.53–11.40) | 0.25 | |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RCCEP, reactive cutaneous capillary endothelial proliferation.
In the Cox regression model that included RCCEP grade as a sequential variable, there was no difference in the PFS or OS between the patients who developed grade 2 and 3 RCCEP after camrelizumab treatment compared to those who developed grade 1 RCCEP (Table 7).
Modified 6-month landmark analysis of patients who developed RCCEP
A modified 6-month landmark survival analysis was conducted for patients with RCCEP, aiming to explore whether the survival difference between early-onset and late-onset RCCEP patients was entirely attributed to the early death of early-onset patients. In this analysis, patients who died within 6 months or developed RCCEP after 6 months were excluded. The reduced sample size of this analysis might have introduced an additional source of bias; however, it helped to alleviate the influence of immortal time bias. No significant association between RCCEP time and PFS or OS (Table 8) was found in the univariate Cox regression analyses.
Table 8
| Variables | PFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Time to RCCEP | |||||
| ≤1 month | Reference | Reference | |||
| >1 and ≤2 months | 0.42 (0.06–3.01) | 0.39 | 0.38 (0.05–2.68) | 0.33 | |
| >2 and ≤3 months | 0.80 (0.15–4.37) | 0.80 | 0.70 (0.13–3.82) | 0.68 | |
| >3 and ≤5 months | 1.36 (0.26–7.04) | 0.71 | 1.23 (0.24–6.38) | 0.80 | |
| >5 and ≤6 months | 1.81 (0.16–19.98) | 0.63 | 1.37 (0.12–15.15) | 0.80 | |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RCCEP, reactive cutaneous capillary endothelial proliferation.
Prognostic factors predicting RCCEP
The risk factors of RCCEP were determined through logistic regression analysis. The variables with a P value ≤0.10 in the univariate logistic analysis (i.e., smoking history, LDH level, treatment cycles, albumin, the PLR, and the neutrophil count) were included in the multivariate logistic regression analysis, which showed that more camrelizumab treatment cycles were independently associated with a higher risk of RCCEP [odds ratio (OR) =1.24; 95% CI: 1.16–1.31; P<0.001], and camrelizumab combined with an anti-angiogenic agent was independently associated with a lower risk of RCCEP (OR =0.24; 95% CI: 0.07–0.86; P=0.03) (Table 9).
Table 9
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 1.01 (0.98–1.03) | 0.65 | |||
| Gender (vs. female) | |||||
| Male | 0.72 (0.45–1.14) | 0.16 | |||
| ECOG PS (vs. <2) | |||||
| ≥2 | 0.76 (0.31–1.86) | 0.55 | |||
| Family history of cancer (vs. ≤no) | |||||
| Yes | 1.39 (0.88–2.20) | 0.16 | |||
| Histology (vs. squamous cell carcinoma) | |||||
| Other | 0.69 (0.18–2.61) | 0.59 | |||
| Metastasis (vs. no) | |||||
| Yes | 0.91 (0.60–1.40) | 0.67 | |||
| Drinking history (vs. no) | |||||
| Yes | 1.02 (0.67–1.56) | 0.92 | |||
| Smoking history (vs. no) | |||||
| Yes | 0.68 (0.45–1.04) | 0.07 | |||
| LDH level (vs. ≤ ULN) | |||||
| > ULN | 0.27 (0.06–1.19) | 0.08 | |||
| Cycles (vs. ≤3) | |||||
| >3 | 1.23 (1.16–1.30) | <0.001* | 1.24 (1.16–1.31) | <0.001* | |
| Antiangiogenic therapy (vs. no) | |||||
| Yes | 0.27 (0.08–0.92) | 0.04* | 0.24 (0.07–0.86) | 0.03* | |
| Radiation (vs. no) | |||||
| Yes | 1.12 (0.74–1.71) | 0.59 | |||
| Line of therapy (vs. 1st) | |||||
| ≥ 2nd | 0.67 (0.41–1.12) | 0.12 | |||
| BMI (kg/m2) (vs. <18.5) | |||||
| ≥18.5 | 1.30 (0.68–2.50) | 0.43 | |||
| Albumin (g/L) (vs. ≤36.8) | |||||
| >36.8 | 3.38 (1.48–7.73) | 0.004 | |||
| PNI (vs. <45.7) | |||||
| ≥45.7 | 1.40 (0.90–2.17) | 0.14 | |||
| NLR (vs. ≤5) | |||||
| >5 | 0.76 (0.46–1.26) | 0.29 | |||
| PLR (vs. ≤152) | |||||
| >152 | 0.58 (0.38–0.90) | 0.02* | |||
| Neutrophil (×109/L) | 0.87 (0.78–0.97) | 0.02* | |||
*, represents P<0.05. BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; RCCEP, reactive cutaneous capillary endothelial proliferation; ULN, upper limit of normal.
Discussion
In this retrospective study, 32.24% of the EC patients treated with camrelizumab developed RCCEP, but the symptoms of RCCEP were mild in most patients. The incidence rate of irTAEs was 11.59%, while that of other irAEs was <5%. A positive correlation was found between the occurrence of irAEs and the efficacy of camrelizumab in the treatment of EC, and the treatment efficacy of the RCCEP patients was better than that of the irAE patients without RCCEP. In patients with RCCEP, there were no significant differences in OS and PFS among individuals classified according to the timing or grading of RCCEP occurrence. More camrelizumab treatment cycles and not receiving combined anti-angiogenic therapy were independent risk factors for RCCEP.
In this study, the incidence rate of RCCEP was 32.24%, which was lower than that of 79.9% reported in the ESCORT-1st study (6). A previous study reported that the occurrence rate of RCCEP after two cycles of standard chemotherapy combined with camrelizumab was 21.1% (14). In the NIC-ESCC2019 study, the occurrence rate of RCCEP after two cycles of neoadjuvant therapy was only 9% (13). In a retrospective study on camrelizumab treatment for NSCLC, for the patients having received at least two cycles of camrelizumab combined therapy, the incidence rate of RCCEP was only 30% (9). Therefore, the number of treatment cycles of camrelizumab was associated with the incidence of RCCEP. A large-scale pooled analysis of 10 studies in China found that the incidence rate of RCCEP was higher in patients using camrelizumab alone (77%) than in patients using combined chemotherapy (67.8%), and both these incidence rates were higher than the incidence rate of patients using combined anti-angiogenic drugs (23.6%) (24). In a study of nasopharyngeal cancer, the incidence of RCCEP with camrelizumab monotherapy was 88% (82/93), whereas the incidence of RCCEP with camrelizumab in combination with gemcitabine/cisplatin was reduced to 22% (5/23) (25). Therefore, the types of drugs used in combination therapy with camrelizumab were associated with the incidence of RCCEP. In the present study, most patients received one or two cycles of camrelizumab treatment, accounting for 17.88% and 17.38%, respectively. However, the patients who received three treatment cycles had the highest incidence rate of RCCEP [31 patients (7.81%)]. After excluding patients who received less than three cycles of therapy and those who received anti-angiogenic therapy, the incidence rate of RCCEP increased to 48.71% (113/232). Unlike standard treatment with only paclitaxel, albumin-bound paclitaxel, or docetaxel combined with platinum-based drugs, camrelizumab was combined with multiple chemotherapy agents in this study, and some patients received underlying disease treatment during anti-tumor therapy. Additionally, various factors such as patients’ economic status, transportation barriers, and adverse drug reactions may affect the continuity of camrelizumab treatment in real-world studies, which can also affect the incidence rate of RCCEP. Further, limitations in real-world research, such as incomplete electronic medical records, a failure to report some minor lesions by patients, and patient recall bias, can also influence the statistical analysis of the RCCEP incidence rate. Therefore, while the occurrence rate of RCCEP in this study was low, it holds greater clinical reference value, as the results were based on real-world statistics.
The patients with RCCEP had a higher ORR and DCR than those without RCCEP. The survival analysis showed that the RCCEP-positive group had significantly longer OS and PFS than the RCCEP-negative group. Moreover, the OS and PFS of the RCCEP-positive group was significantly longer than the OS and PFS of the RCCEP-negative, irAE-positive group, and the irAE-negative group; the OS and PFS of the RCCEP-negative, irAE-positive group was significantly longer than the OS and PFS of the irAE-negative group. The Cox analysis also indicated that RCCEP was an independent risk factor for both OS and PFS, while irAEs were independent risk factors for OS but not PFS. Therefore, we speculate that RCCEP, a dermatological abnormality, may be associated with longer OS and PFS.
The treatment efficacy of patients with irAEs who did not experience RCCEP was lower than that of those with RCCEP, but they still had better outcomes than the patients without irAEs. However, the relationship between the irAEs other than RCCEP and treatment efficacy could not be individually evaluated due to the small sample size of the study and the low incidence rates. Therefore, we need to collect more samples collected for statistical analysis in the future.
Further, our study found no relationship between RCCEP time and OS or PFS, which is consistent with the results that we obtained in the modified 6-month landmark analysis. However, we only analyzed the RCCEP time on a monthly basis for the first 6 months; thus, it remains unclear whether there is an inflection point between RCCEP time and efficacy after 6 months. Therefore, we need to observe with a larger sample size over a longer period.
Additionally, we found no relationship between RCCEP grade and OS or PFS. However, a systematic review and meta-analysis has shown that low-grade irAEs have a significant predictive effect on treatment efficacy, but this is not the case for severe-grade irAEs (3). There could be two potential explanations for this finding. First, effective ICIs need to be discontinued temporarily or even permanently when severe irAEs occur; however, as most RCCEP lesions are grade 1 or grade 2, camrelizumab does not need to be discontinued (24,26). Conversely, while treatment does need to be suspended for grade 3 RCCEP, when the RCCEP is reduced to grade 1, camrelizumab treatment can be resumed (26). Cases of grade 4 or 5 RCCEP have not been reported (27,28); therefore, it is likely that patients with RCCEP will not experience the reduction in efficacy associated with the discontinuation of camrelizumab. Second, systemic immunosuppressive therapy is required for severe irAEs, which may counteract the effects of ICIs. Glucocorticoids extensively alter cytokine signaling and inhibit the IL-2 and interferon (INF)-γ pathways (29-31) that are reactivated during ICI treatment to create an inflammatory tumor microenvironment, and exposure to large amounts of immunosuppressive agents during high-grade irAEs may alter the anti-tumor effect. However, most cases of RCCEP do not require special treatment, as the majority of lesions spontaneously resolve within 1 to 2 months of the discontinuation of camrelizumab, and the remaining cases can be managed with symptomatic treatments, such as laser therapy, minor excision, hemostasis procedures, local corticosteroid administration, systemic antibiotics usage, and cryotherapy (18). RCCEP is not sensitive to glucocorticoids, and the treatment of RCCEP does not require immunosuppressive reagents, which may be another key reason why the grade of RCCEP was not found to be related to OS or PFS.
The predictive role of RCCEP in the efficacy of EC treatment is consistent with findings for NSCLC and HCC. However, opinions differ as to whether irAEs can serve as predictors for the effectiveness of ICIs in tumor therapy. A significant association between increased survival after ICI treatment and irAEs has been reported for both GI cancer (32) and NSCLC (33). However, it is not yet known whether the occurrence of irAEs is correlated with a superior response and improved survival outcomes in patients with melanoma (34,35). Moreover, studies have shown that the irAEs induced by PD-1 inhibitors are predictive of a better clinical response in patients with cancer, but the association between irAEs and survival in patients undergoing anti-CTLA-4 therapy remains controversial (36-41). According to another study, the type of irAE may also be predictive of ICI efficacy in tumor treatment. Significant survival benefits were observed in patients with endocrine and skin abnormalities, whereas no comparable survival advantages were noted in patients with GI, pulmonary, hepatobiliary, or musculoskeletal abnormalities (3). In studies investigating the relationship between the number of irAEs and treatment efficacy, some have found that a higher number indicates better efficacy (33,42), while others have reached the opposite conclusion (1). Regarding severity grading, some studies reported a correlation between higher grades and good efficacy (43,44), while others have not found evidence of any such correlation (3). In terms of timing, there is research suggesting that patients with late onset irAEs (i.e., irAE onset >3 months) have better outcomes than those with early onset irAEs (i.e., irAE onset ≤3 months) (45). However, there are limited studies on the timing of irAE occurrence, and no conclusive results have been obtained. Such conflicting findings currently prevent us from drawing definitive and unified conclusions. Therefore, we speculate that the predictive role of the site, frequency, severity, and timing of irAE occurrence on treatment efficacy may vary in different tumors treated with different ICIs. In the future, we should consider conducting prospective trials to further explore the relationship between irAEs and the efficacy of cancer treatments.
In this study, more camrelizumab treatment cycles and not receiving combined anti-angiogenic therapy were significantly associated with RCCEP occurrence. The precise mechanism underlying the induction of RCCEP by camrelizumab remains elusive. We hypothesize that camrelizumab disrupts the dynamic balance between angiogenic factors and anti-angiogenic factors (46), resulting in an increased risk of RCCEP. As the treatment regimen with camrelizumab progresses, this disruptive effect persists and intensifies, consequently augmenting the likelihood of RCCEP occurrence. Studies have shown that the incidence of RCCEP was significantly reduced when camrelizumab was combined with anti-angiogenic drugs (9,24,47), which is consistent with the results of this study. It has been suggested that anti-angiogenic drugs may block angiogenesis by targeting the VEGF/VEGF receptor (VEGFR) signaling pathway, thereby further preventing the occurrence of RCCEP (9). To date, no consensus has been reached as to whether the application of anti-angiogenic drugs solely prolongs the onset time of RCCEP or prevents its occurrence (24). This study found that patients with RCCEP had better treatment outcomes than those without RCCEP. However, currently, it is unclear whether there are differences in treatment efficacy between patients who experienced RCCEP after the application of anti-angiogenic drugs and those who did not experience RCCEP, as well as those who should have experienced RCCEP but did not due to the use of anti-angiogenic drugs. Thus, further research with larger sample sizes is needed to be conducted in the future.
There are several limitations in this study that should be addressed. First, as it employed a single-center, retrospective design, bias related to the selection of patients is inevitable, and potential confounding factors could have been left unaccounted for. Second, while our study examined a large volume of data, the sample size and follow-up time for specific indicators were not particularly robust. Third, methodology limitations include potential underreporting of adverse events, and lack of mechanistic exploration into the RCCEP-efficacy relationship. Given these limitations, the findings should be interpreted carefully. Multicenter prospective studies are warranted to validate the association between RCCEP and camrelizumab, and to further analyze the impact of treatment heterogeneity in combination therapies (chemotherapy/anti-angiogenics) on the outcomes. Currently, the underlying mechanism or relationship between RCCEP and therapeutic efficacy remains unclear, necessitating additional studies for in-depth investigation.
Conclusions
In EC patients treated with camrelizumab, those with RCCEP experienced significantly better outcomes in terms of the ORR, DCR, PFS, and OS than those without RCCEP. The patients who developed irAEs other than RCCEP did less well than the RCCEP-positive patients, but better than the patients without irAEs, and no correlation was found between the duration and severity of RCCEP and prolonged PFS or OS. More camrelizumab treatment cycles and not receiving combined anti-angiogenic therapy were independent risk factors for RCCEP. These findings may provide a basis for clarifying the mechanism underlying RCCEP.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-366/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-366/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-366/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-366/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Ethical approval for the current research was granted by the Research Ethics Committee of The Fourth Hospital of Hebei Medical University (approval No. 2022KS014). The requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qin W, Yang L, Fan B, et al. Association between immune-related adverse events and the efficacy of PD-1 inhibitors in advanced esophageal cancer. Front Immunol 2022;13:931429. [Crossref] [PubMed]
- Zhou P, Liu B, Shen N, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors: a single-center retrospective study. Ren Fail 2024;46:2326186. [Crossref] [PubMed]
- Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18:87. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. [Crossref] [PubMed]
- Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 2018;363:k4226. [Crossref] [PubMed]
- Yang J, He X, Lv Q, et al. Management of Adverse Events in Cancer Patients Treated With PD-1/PD-L1 Blockade: Focus on Asian Populations. Front Pharmacol 2019;10:726. [Crossref] [PubMed]
- He X, Fang J, Yu P, et al. Risk of reactive cutaneous capillary endothelial proliferation induced by camrelizumab in patients with non-small cell lung cancer: a retrospective study. J Thorac Dis 2023;15:6687-96. [Crossref] [PubMed]
- Huang G, Liu S, Dong J, et al. PD-1 inhibitor-based adverse events in solid tumors: A retrospective real-world study. Front Pharmacol 2022;13:974376. [Crossref] [PubMed]
- He M, Wang Z, Lu J, et al. Final analysis of camrelizumab plus chemotherapy for untreated advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st trial. Med 2024;5:1137-1149.e3. [Crossref] [PubMed]
- Yang Y, Zhang J, Meng H, et al. Neoadjuvant camrelizumab combined with paclitaxel and nedaplatin for locally advanced esophageal squamous cell carcinoma: a single-arm phase 2 study (cohort study). Int J Surg 2024;110:1430-40. [Crossref] [PubMed]
- Liu J, Li J, Lin W, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer 2022;151:128-37. [Crossref] [PubMed]
- Zhou RQ, Luo J, Li LJ, et al. Neoadjuvant camrelizumab plus chemotherapy in locally advanced oesophageal squamous cell carcinoma: a retrospective cohort study. BMC Surg 2023;23:114. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Lickliter JD, Gan HK, Voskoboynik M, et al. A First-in-Human Dose Finding Study of Camrelizumab in Patients with Advanced or Metastatic Cancer in Australia. Drug Des Devel Ther 2020;14:1177-89. [Crossref] [PubMed]
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol 2016;74:455-61.e1. [Crossref] [PubMed]
- Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol 2020;13:47. [Crossref] [PubMed]
- Hu Y, Shen J, Liu R, et al. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: A systematic review and meta-analysis. Int J Biol Markers 2018;33:372-8. [Crossref] [PubMed]
- Liu J, Hu G, Zhai C, et al. Predictive value of nutritional indicators with regard to the survival outcomes in patients with metastatic esophageal squamous cell carcinoma treated with camrelizumab. Oncol Lett 2023;25:198. [Crossref] [PubMed]
- Chen W, Li D, Bian X, et al. Peripheral Blood Markers Predictive of Progression-Free Survival in Advanced Esophageal Squamous Cell Carcinoma Patients Treated With PD-1 Inhibitors Plus Chemotherapy as First-Line Therapy. Nutr Cancer 2023;75:207-18. [Crossref] [PubMed]
- Chen N, Yu Y, Shen W, et al. Nutritional status as prognostic factor of advanced oesophageal cancer patients treated with immune checkpoint inhibitors. Clin Nutr 2024;43:142-53. [Crossref] [PubMed]
- Feng JF, Wang L, Chen QX, et al. Development and Validation of a New Integrative Score Based on Various Systemic Inflammatory and Nutritional Indicators in Predicting Prognosis in Patients With Resectable Esophageal Squamous Cell Carcinoma: A Retrospective Cohort Study. Cancer Control 2022;29:10732748221091394. [Crossref] [PubMed]
- Qu W, Wang F, Qin S, et al. Reactive cutaneous capillary endothelial proliferation following camrelizumab monotherapy or combination therapy for multi-cancers: a large-scale pooled analysis of 10 studies in China. Ther Adv Med Oncol 2024;16:17588359241242607. [Crossref] [PubMed]
- Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol 2018;19:1338-50. [Crossref] [PubMed]
- Hui-Mei P, Guang-Ming H, Xiao-Ling Q, et al. Reactive Cutaneous Capillary Endothelial Proliferation Caused by Camrelizumab: Sixteen Case Reports. Indian J Dermatol 2023;68:318-26. [Crossref] [PubMed]
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol 2020;83:1255-68. [Crossref] [PubMed]
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol 2021;39:4073-126. [Crossref] [PubMed]
- Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens 2006;68:1-12. [Crossref] [PubMed]
- Hu X, Li WP, Meng C, et al. Inhibition of IFN-gamma signaling by glucocorticoids. J Immunol 2003;170:4833-9. [Crossref] [PubMed]
- Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci U S A 2000;97:9573-8. [Crossref] [PubMed]
- Masuda K, Shoji H, Nagashima K, et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 2019;19:974. [Crossref] [PubMed]
- Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 2019;145:479-85. [Crossref] [PubMed]
- Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019;145:511-21. [Crossref] [PubMed]
- Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785-92. [Crossref] [PubMed]
- Lang N, Dick J, Slynko A, et al. Clinical significance of signs of autoimmune colitis in (18)F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy 2019;11:667-76. [Crossref] [PubMed]
- Sachpekidis C, Larribère L, Kopp-Schneider A, et al. Can benign lymphoid tissue changes in (18)F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol Immunother 2019;68:297-303. [Crossref] [PubMed]
- Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706-14. [Crossref] [PubMed]
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33:773-81. [Crossref] [PubMed]
- Wong ANM, McArthur GA, Hofman MS, et al. The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur J Nucl Med Mol Imaging 2017;44:67-77. [Crossref] [PubMed]
- Di Giacomo AM, Calabrò L, Danielli R, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 2013;62:1021-8. [Crossref] [PubMed]
- Shimozaki K, Sukawa Y, Beppu N, et al. Multiple Immune-Related Adverse Events and Anti-Tumor Efficacy: Real-World Data on Various Solid Tumors. Cancer Manag Res 2020;12:4585-93. [Crossref] [PubMed]
- Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J Clin Oncol 2019;37:2730-7. [Crossref] [PubMed]
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. [Crossref] [PubMed]
- Hsiehchen D, Naqash AR, Espinoza M, et al. Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology 2022;11:2017162. [Crossref] [PubMed]
- Wang R, Shi M, Ji M, et al. Real world experience with camrelizumab in patients with advanced non-small cell lung cancer: a prospective multicenter cohort study (NOAH-LC-101). Transl Lung Cancer Res 2023;12:786-96. [Crossref] [PubMed]
- Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res 2019;25:515-23. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



