
Computed tomography characteristics of chronic bronchitis and its association with disease severity and clinical outcomes in viral pneumonia: a retrospective cohort study
Highlight box
Key findings
• The pathological characteristics of chronic bronchitis (CB), including mucus hypersecretion, emphysema, and wall thickening, were found to be key factors that were associated with greater severity of viral pneumonia and worse clinical outcomes.
What is known and what is new?
• The pathological characteristics of CB include mucus hypersecretion, emphysema, and wall thickening.
• In this study, mucus hypersecretion in patients with CB was identified as a factor correlated with a greater severity of viral pneumonia and worse clinical outcomes.
What is the implication, and what should change now?
• This study examined the characteristics of CB in the context of mucus hypersecretion, emphysema, and bronchial wall thickening, with the aim of characterizing the manifestations of CB within the framework of viral lung infections. Moreover, we identified critical factors that aggravate viral pneumonia and potentially influence its clinical outcomes, thereby offering novel insights and evidence for the diagnosis and management of CB. The special pathological characteristics of CB and their implications suggest that clinicians should confirm the diagnosis as early as possible. After diagnosis, measures such as controlling symptoms, preventing infections, and administering appropriate medication should be applied.
Introduction
Viral respiratory infections are the primary cause of acute respiratory diseases in healthy individuals across all age groups. Common viruses associated with these infections include influenza, respiratory syncytial virus, parainfluenza, and severe acute respiratory syndrome coronavirus (SARS-CoV). Upon invading the respiratory tract, these viruses promptly activate the innate defense response of the airway epithelium by stimulating macrophages, epithelial cells, and dendritic cells (1). However, the specific pathogenesis leading to abnormal mucus secretion in the airways due to viral infections remains unclear. Some research suggests that severe coronavirus infection may trigger a cytokine release syndrome, initiating a proinflammatory cascade that results in excessive mucus production by the infected respiratory epithelial cells. This disrupts the mucociliary clearance function, further causing airway obstruction and dyspnea (2). Airway mucus hypersecretion is a pathophysiological process in which various pathogenic factors induce excessive mucus production in the airway mucosa. This condition is caused by multiple pathogenic factors, leading to abundant secretions from cells within the airway, which stimulate the hyperplasia and hypertrophy of goblet cells and submucosal glands in the mucosa, ultimately resulting in mucus overproduction. A previous study (3) has shown that excessive airway mucus secretion also occurs in acute respiratory infections, such as those involving coronaviruses, and that it is a significant risk factor for the onset, progression, and prognosis of airway inflammation.
Chronic bronchitis (CB) is characterized by a persistent productive cough lasting more than 3 months and recurring over 2 consecutive years (4). The pathogenic factors contributing to CB encompass inhalation of respiratory irritants, recurrent bacterial and/or viral infections, preexisting chronic respiratory diseases, and continual exposure to environmental pollutants. The pathophysiology of CB involves an excessive production and secretion of mucus by goblet cells, resulting in a distinctive cough. This condition aggravates airflow obstruction and deteriorates lung function. An epidemiological study (5) has demonstrated that individuals with CB are at a significantly increased risk of developing new-onset chronic obstructive pulmonary disease and exhibit a higher mortality rate. The characteristic pathological alterations in CB—including goblet cell hyperplasia, mucus hypersecretion, and subsequent mechanical obstruction of small airways, airway structural remodeling, and abnormal surface tension—would lead to adverse clinical outcomes such as accelerated decline in pulmonary function and heightened susceptibility to lower respiratory tract infections (6).
However, the mechanisms underlying viral infection susceptibility in CB patients, as well as the associations between computed tomography (CT)-derived phenotypes and clinical prognosis, remain poorly defined. Crucially, there is a lack of validated CT-based quantitative parameters for stratifying post-viral pneumonia severity in this population.
The objective of this study was to investigate the association of CB and its related CT imaging characteristics with the severity and clinical outcomes of viral pneumonia. The purpose of this study was to provide a foundation for personalized clinical treatment plans following viral infection, with the ultimate goal of reducing the adverse effects of acute respiratory infections. We hypothesized that CB and its characteristics increase the severity of viral pneumonia, leading to more severe clinical manifestations and poorer clinical outcomes. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-638/rc).
Methods
Study design and participants
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study received approval from the Ethics Committee for Human Research at Ningbo No. 2 Hospital (Ningbo, Zhejiang, China; approval No. YJ-NBEY-KY-2024-046-01). Informed consent was taken from all the patients. We retrospectively collected data from consecutively admitted patients meeting inclusion criteria between December 2022 and December 2024 at Ningbo No. 2 Hospital. Random sampling was not employed given the observational cohort design. Participants were stratified into two cohorts by mucus plugging score: high mucus burden (mucus plugging score ≥4, group 1) and low mucus burden (mucus plugging score <4, group 2). The patients were further stratified into CB and non-CB groups based on whether they met the diagnostic criteria for CB [post-bronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <0.7 with chronic cough and sputum production for ≥3 months per year over ≥2 consecutive years]. The inclusion criteria were as follows: age 18 years or older, confirmed etiological diagnosis of viral pneumonia, availability of complete clinical data and initial and follow-up chest CT images, and willingness to provide informed consent. Meanwhile, the exclusion criteria were as follows: a history of lung cancer or related treatment, diagnosis of infection with other pathogens or noninfectious diseases (e.g., drug-induced lung inflammation), and incomplete data or poor CT image quality (Figure 1). Baseline covariates encompassed demographic characteristics (age, sex, smoking history) and laboratory parameters [red blood cell count (RBC), white blood cell count (WBC), hemoglobin (Hb), and C-reactive protein (CRP)]. CB severity was systematically evaluated using CT severity scores and quantitative imaging biomarkers, including consolidation, ground-glass opacities (GGOs), reticular patterns, emphysema, Pi10 (the square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm, standardized metrics reflecting airway wall thickness), and bronchial-related metrics (e.g., lumen diameter, wall area%). Primary outcomes focused on radiographic prognosis, defined by longitudinal changes in CT severity scores and the temporal evolution of specific imaging features (e.g., resolution of consolidation, progression of GGO).
CT image acquisition and interpretation
All participants underwent an initial CT scan upon admission. A follow-up CT was conducted within a 3-month period with a 96-slice, dual-source, dual-detector scanner (SOMATOM Force, Siemens Healthineers, Erlangen, Germany). The selection of this 3-month interval for follow-up was grounded on comprehensive clinical experience, as this duration offers an adequate observation window to evaluate the trajectory of a patient’s recovery.
During the CT procedure, patients were directed to assume a relaxed bodily posture and maintain breath-holding at the peak of deep inhalation. The scanning coverage encompassed the area from the apex of the thorax to the plane of the bilateral diaphragmatic surfaces. The specific scanning parameters were as follows: tube voltage, 100–130 kV; an automated tube current; pitch setting, 1.2; the gantry rotation time, 2.88 seconds; collimation, 0.6 mm × 192 mm; slice thickness, 5 mm; and image-reconstruction slice thickness, 1 mm.
All 260 patients underwent follow-up CT scans with the same scanner employed in their initial examination. Two senior cardiothoracic radiologists who were blinded to clinical or laboratory findings and patient outcomes reviewed all CT images in a random order. Each lung segment was systematically and independently examined by the two radiologists for the presence of mucous plugs, with a score of 1 or 0 assigned accordingly. The scores were then summed, with the 18 lung segments in both lungs being considered, resulting in a total score ranging from 0 to 18. Mucus plugs were defined as foci of heterogeneous density that completely obstructed the airway lumen that had no correlation with airway size or generation, with lesions within 2 cm of the parietal or diaphragmatic pleura being excluded. The final scores from both raters were averaged to generate a CT mucus score for each participant (7) (Figure 2). The mean values of the two radiologists’ assessments were calculated. Patients were divided into the following two groups according to a mucus score: the high mucus burden group (score ≥4; group 1) and the low mucus burden group (score <4; group 2).

For each patient, the primary CT patterns (8) were identified as interleukin-6 (IL-6), GGO, consolidation, reticulation, and emphysema, in accordance with the Fleischner Society glossary. The bronchial wall and lumen volumes for all patients were evaluated. To assess the severity of pulmonary lesions evident in the imaging results, two radiologists semi-quantitatively scored all CT images based on the affected area within each of the lung’s five lobes. The scoring scheme is as follows: 0, no involvement; 1, less than 5% involvement; 2, 5–25% involvement; 3, 26–49% involvement; 4, 50–75% involvement; and 5, more than 75% involvement. The overall CT score was derived by adding the scores from each individual lobe, resulting in a range of 0 to 25 (9) (Figure 3).

Statistical analysis
Statistical analyses were conducted using SPSS version 27.0 (IBM Corporation, Armonk, NY, USA). Missing values were imputed via the median. Data that were normally distributed were presented as mean and standard deviation (SD), while nonnormally distributed data were presented as the median and interquartile range (IQR). Categorical data were presented as the number and percentage. Continuous variables that met the criteria of normality and homogeneity of variance tests were expressed as the mean ± SD, and the t-test was employed for intergroup comparisons. Conversely, data not adhering to a normal distribution were presented as the median and IQR, with the Mann-Whitney test being used for intergroup comparisons. Categorical variables underwent descriptive analysis using frequency and percentage [n (%)]. The choice of nonparametric tests was determined based on factors such as theoretical frequency and other relevant criteria. Logistic regression analysis was conducted to identify independent risk factors for CB patient classification and evaluate the diagnostic performance of the predictive model. All statistical tests were two-sided, with a P value <0.05 considered statistically significant.
Results
Demographic information and patient characteristics
This study enrolled 260 patients who were categorized into group 1 (n=42; CB prevalence: 35.7%) and group 2 (n=218; CB prevalence: 19.7%) (Figure 4). Patients diagnosed with CB were significantly older (P<0.001) and more likely to be female (P=0.03) compared to those without CB. Patients with CB had a lower RBC count (P=0.02) and Hb levels (P=0.04), but no significant difference was found in white blood cell count. No significant differences were observed in mucus score, IL-6 level, or other indicators between the two groups. Although there were no significant differences in sex, white blood cell count, RBC count, Hb level, or mucus score (all P values >0.05), age was significantly different in patients in group 1 (Table 1).

Table 1
| Characteristics | Patients without CB | Patients with CB | t/Z value | P value |
|---|---|---|---|---|
| Sex | ||||
| G1 | 0.05 | 0.82 | ||
| M | 12 (44.44) | 8 (53.33) | ||
| F | 15 (55.56) | 7 (46.47) | ||
| G2 | 4.96 | 0.03* | ||
| M | 80 (45.71) | 11 (25.58) | ||
| F | 95 (54.29) | 32 (74.42) | ||
| Age (years) | ||||
| G1 | 77 [72.5, 82] | 83 [77.5, 87] | −1.79 | 0.08 |
| G2 | 70 [62, 77] | 79 [74.5, 86.5] | −4.71 | <0.001*** |
| Smoking history | ||||
| G1 | 0.01 | 0.01* | ||
| Never-smoker | 5 (18.52) | 2 (13.33) | ||
| Ex-smoker | 8 (29.63) | 3 (20.00) | ||
| Current smoker | 14 (51.85) | 10 (66.67) | ||
| G2 | 9.47 | 0.002** | ||
| Never-smoker | 20 (11.43) | 5 (11.63) | ||
| Ex-smoker | 45 (25.71) | 8 (18.60) | ||
| Current smoker | 110 (62.86) | 30 (69.77) | ||
| WBC | ||||
| G1 | 7.80 [6.00, 9.10] | 6.20 [5.35, 8.80] | 0.96 | 0.34 |
| G2 | 6.50 [4.80, 7.70] | 6.50 [5.15, 9.50] | −0.97 | 0.33 |
| RBC | ||||
| G1 | 4.03 [3.51, 4.38] | 3.78 [3.39, 4.16] | 0.84 | 0.41 |
| G2 | 4.05 [3.81, 4.53] | 3.73 [3.39, 4.29] | 2.43 | 0.02* |
| Hb | ||||
| G1 | 122 [107, 132.5] | 121 [102, 124] | 0.91 | 0.37 |
| G2 | 124 [115.5, 136] | 119 [105.5, 131] | 2.04 | 0.04* |
| Mucus score | ||||
| G1 | 6.00 [4.75, 7.00] | 6.00 [4.75, 7.00] | −0.09 | 0.94 |
| G2 | 1.50 [0.50, 2.00] | 2.00 [0.50, 2.50] | −0.92 | 0.36 |
| CT score | ||||
| G1 | 10.00 [6.50, 12.50] | 8.00 [7.00, 9.500] | 1.10 | 0.27 |
| G2 | 5.00 [0.00, 8.00] | 6.00 [1.50, 10.00] | −1.67 | 0.09 |
Data are presented as n (%) or median [interquartile range]. G1: group 1, high mucus group; score ≥4. G2: group 2, low mucus group; score <4. *, P<0.05; **, P<0.01; ***, P<0.001. CB, chronic bronchitis; CT, computed tomography; F, female; Hb, hemoglobin; M, male; RBC, red blood cell count; WBC, white blood cell count.
Comparison of initial CT findings in groups 1 and 2
In group 2 (Table 2), patients with CB had significantly higher emphysema volumes (P=0.002) after pulmonary viral infections, as well as significantly lower luminal volume (P<0.001). In addition, more severe reticulation changes were observed on chest CT images in patients with CB (P=0.02), while no significant differences were found between the two groups in terms of low attenuation area (LAA; used to quantify emphysema severity) or Pi10 indices. Although there were no significant differences in LAA, LAA percentage (LAA%), reticulation, GGO, consolidation, or Pi10 indices between the two groups, statistically significant differences in emphysema in group 2 and wall volume among patients were observed in both groups.
Table 2
| Characteristics | Patients with CB | Patients without CB | t/Z value | P value |
|---|---|---|---|---|
| LAA% | ||||
| G1 | 0.59 [0.20, 1.78] | 0.80 [0.19, 1.35] | −0.03 | 0.99 |
| G2 | 1.46 [0.26, 6.64] | 1.85 [0.15, 6.02] | 0.13 | 0.99 |
| Reticulation | ||||
| G1 | 7.07 [3.50, 12.02] | 6.28 [3.69, 11.23] | −0.07 | 0.96 |
| G2 | 2.50 [0.57, 6.19] | 6.02 [1.42, 10.08] | −2.33 | 0.02* |
| GGO | ||||
| G1 | 13.85 [5.2, 2.17] | 8.53 [4.22, 15.48] | 1.011 | 0.32 |
| G2 | 2.18 [0.67, 6.93] | 2.73 [0.99, 12.37] | −0.99 | 0.32 |
| Consolidation | ||||
| G1 | 0.25 [0.07, 0.54] | 0.18 [0.12, 0.29] | 0.709 | 0.49 |
| G2 | 0.09 [0.02, 0.27] | 0.09 [0.03, 0.24] | −0.30 | 0.76 |
| Emphysema | ||||
| G1 | 0.06 [0.02, 0.12] | 0.29 [0.06, 0.90] | −1.89 | 0.06 |
| G2 | 0.05 [0.01, 0.19] | 0.21 [0.04, 0.73] | −3.12 | 0.002** |
| Pi10 | ||||
| G1 | 4.77 [3.98, 5.32] | 4.90 [1.64, 5.42] | 0.38 | 0.71 |
| G2 | 4.25 [3.44, 5.00] | 4.19 [2.61, 5.09] | 0.66 | 0.51 |
| Wall volume | ||||
| G1 | 21.60 [10.30, 37.69] | 6.28 [1.54, 15.71] | 2.24 | 0.03* |
| G2 | 35.08 [13.38, 51.59] | 18.90 [3.43, 45.53] | 2.50 | 0.01* |
| Lumen volume | ||||
| G1 | 5.29 [2.69, 12.30] | 4.57 [1.02, 12.26] | 0.55 | 0.59 |
| G2 | 13.63 [5.19, 25.29] | 5.00 [0.67, 13.46] | 3.95 | <0.001*** |
Data are presented as median [interquartile range]. G1: group 1, high mucus group; score ≥4. G2: group 2, low mucus group; score <4. *, P<0.05; **, P<0.01; ***, P<0.001. CB, chronic bronchitis; CT, computed tomography; GGO, ground-glass opacity; LAA, low attenuation area.
Univariate analysis of the two groups after lung viral infection
Age was found to be a significant factor affecting the probability of CB (P=0.05) in group 1, and the risk of CB increased with age [odds ratio (OR) =1.10], but the actual impact range was small [95% confidence interval (CI): 1.00–1.22]. In group 2, univariate analysis showed that sex (P=0.02) and age (P<0.001) were significantly associated with the classification of patients with CB. Among hematological indicators, RBC count (P=0.02) and Hb level (P=0.04) were lower in patients with CB. In terms of chest CT imaging features, emphysema (P=0.005) was a significant factor in patients with CB. The decrease in wall volume (P=0.02) and the significant decrease in luminal volume (P=0.002) were significantly associated with the classification of patients with CB. The negative effect of luminal volume (OR <1) was more pronounced, indicating that the decrease in luminal volume was a strong predictor of CB (Table 3).
Table 3
| Characteristics | B | Standard error | Wald | Degrees of freedom | P value | OR | 95% CI of OR | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| G1 | ||||||||
| Age | 0.10 | 0.05 | 3.99 | 1 | 0.046* | 1.10 | 1.00 | 1.22 |
| RBC | −0.38 | 0.54 | 0.47 | 1 | 0.49 | 0.69 | 0.24 | 1.98 |
| Mucus score | 0.06 | 0.18 | 0.09 | 1 | 0.76 | 1.06 | 0.74 | 1.51 |
| Emphysema | 0.98 | 0.69 | 2.04 | 1 | 0.15 | 2.66 | 0.69 | 10.17 |
| Pi10 | −0.12 | 0.15 | 0.61 | 1 | 0.44 | 0.89 | 0.67 | 1.12 |
| Wall volume | −0.02 | 0.02 | 1.55 | 1 | 0.21 | 0.98 | 0.95 | 1.01 |
| Lumen volume | −0.01 | 0.03 | 0.01 | 1 | 0.92 | 0.99 | 0.95 | 1.05 |
| G2 | ||||||||
| Sex | 0.90 | 0.38 | 5.53 | 1 | 0.02* | 2.45 | 1.16 | 5.17 |
| Age | 0.08 | 0.02 | 19.20 | 1 | <0.001*** | 1.08 | 1.04 | 1.12 |
| RBC | −0.54 | 0.23 | 5.72 | 1 | 0.02* | 0.58 | 0.37 | 0.91 |
| Hb | −0.02 | 0.01 | 4.13 | 1 | 0.042* | 0.98 | 0.97 | 0.99 |
| Emphysema | 0.49 | 0.17 | 7.96 | 1 | <0.001** | 1.62 | 1.16 | 2.28 |
| Wall volume | −0.02 | 0.01 | 5.22 | 1 | 0.02* | 0.98 | 0.97 | 0.99 |
| Lumen volume | −0.05 | 0.02 | 9.47 | 1 | 0.002** | 0.95 | 0.92 | 0.98 |
G1: group 1, high mucus group; score ≥4. G2: group 2, low mucus group; score <4. *, P<0.05; **, P<0.01; ***, P<0.001. CI, confidence interval; Hb, hemoglobin; OR, odds ratio; RBC, red blood cell count.
Multivariate analysis and receiver operating characteristic (ROC) curve analysis for all patients in group 2 after lung viral infection
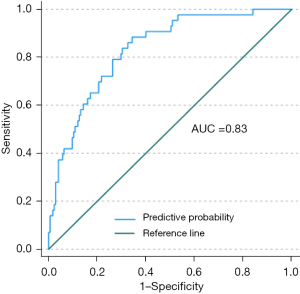
Multivariate analysis revealed significant associations between CB patient classification and sex, age, and emphysema in group 2. Specifically, sex (P=0.009) was significantly associated with CB patient classification, with female patients at higher risk (OR =3.39). Age (P=0.004) was also a significant risk factor, with the risk of CB increasing with age (OR =1.06). Emphysema (P=0.005) remained significant in multivariate analysis, suggesting its status as an independent risk factor for CB. Notably, although luminal volume approached significance in the multivariate model (P=0.06), its effect was relatively small (OR =0.96) and the lower limit of the CI was close to 1, suggesting that although luminal volume may exert a degree of influence on the classification of patients with CB, its effect is limited (Table 4). ROC curve analysis showed that when the optimal cutoff value for emphysema was 0.15, the sensitivity of emphysema was 0.88 and the specificity was 0.66 (Table 5). The mean area under the curve (AUC) for all variables was 0.83. According to the established diagnostic discrimination benchmarks (e.g., Hosmer-Lemeshow criteria), AUC values of 0.7–0.9 indicate moderate to strong predictive utility, aligning with the observed performance of the included variables (Figure 5).
Table 4
| Characteristics | B | Standard error | Wald | Degrees of freedom | P value | OR | 95% CI of OR | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Sex | 1.22 | 0.47 | 6.81 | 1 | 0.009** | 3.39 | 1.35 | 8.50 |
| Age | 0.06 | 0.02 | 8.38 | 1 | 0.004** | 1.06 | 1.02 | 1.10 |
| RBC | −0.61 | 0.90 | 0.45 | 1 | 0.50 | 0.55 | 0.09 | 3.19 |
| Hb | 0.00 | 0.03 | 0.00 | 1 | >0.99 | 1.00 | 0.94 | 1.06 |
| Emphysema | 0.44 | 0.16 | 7.78 | 1 | 0.005** | 1.56 | 1.14 | 2.13 |
| Wall volume | −0.01 | 0.01 | 1.31 | 1 | 0.25 | 0.99 | 0.97 | 1.01 |
| Lumen volume | −0.04 | 0.02 | 3.48 | 1 | 0.06 | 0.96 | 0.92 | 1.01 |
Group 2, low mucus group; score <4. **, P<0.01. CI, confidence interval; Hb, hemoglobin; OR, odds ratio; RBC, red blood cell count.
Table 5
| Characteristics | AUC | 95% CI | Optimal threshold | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Emphysema | 0.83 | 0.77 | 0.89 | 0.15 | 0.88 | 0.66 |
Group 2, low mucus group; score <4. AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
Mann-Whitney test for initial and follow-up CT
A significant difference was observed in the initial CRP level between patients with and without CB in group 1 (P=0.002). The median CRP level of patients without CB was significantly higher than that of patients with CB, suggesting a more pronounced inflammatory response in patients without CB. However, this difference was not significant at follow-up. A comparison of re-examination indicators among all patients in group 2 revealed significant differences in RBC count and Hb level, indicating a potential tendency toward anemia in patients with CB. Additionally, although the changes in platelet count did not reach statistical significance, a slight increase in platelets was observed after re-examination (P=0.07), potentially indicating a compensatory response. It is worth noting that the levels of inflammatory markers, including CRP, leukocytes, and IL-6, as well as their levels at follow-up, were not significantly different between the two groups (Table 6).
Table 6
| Characteristics | Patients without CB | Patients with CB | U value | Z value | P value |
|---|---|---|---|---|---|
| CRP | |||||
| Initial | 37.50 [15.03, 69.63] | 63.60 [17.19, 82.84] | 3,183.00 | −1.56 | 0.12 |
| Follow-up | 9.080 [2.30, 37.34] | 14.94 [4.13, 33.66] | 3,508.50 | −0.69 | 0.49 |
| WBC | |||||
| Initial | 6.50 [4.80, 7.70] | 6.50 [5.15, 9.50] | 3,403.00 | −0.97 | 0.33 |
| Follow-up | 7.30 [5.75, 8.70] | 7.40 [5.85, 9.40] | 3,559.00 | −0.55 | 0.58 |
| RBC | |||||
| Initial | 4.05 [3.81, 4.53] | 3.73 [3.39, 4.29] | 4,664.50 | 2.43 | 0.02* |
| Follow-up | 3.90 [3.50, 4.38] | 3.78 [3.27, 4.02] | 4,739.50 | 2.65 | 0.008** |
| Hb | |||||
| Initial | 124 [115, 136] | 119 [105, 131] | 4,516.50 | 2.04 | 0.04* |
| Follow-up | 119 [108, 133] | 112 [101, 125] | 4,575.00 | 2.19 | 0.03* |
| PLT | |||||
| Initial | 200 [148, 248] | 179 [133, 247] | 4,098.50 | 0.91 | 0.37 |
| Follow-up | 216 [169, 309] | 213 [160, 249] | 4,442.00 | 1.83 | 0.07 |
| IL-6 | |||||
| Initial | 132.20 [27.77, 132.20] | 132.20 [15.13, 132.20] | 3,988.50 | 0.61 | 0.48 |
| Follow-up | 132.20 [132.20, 132.20] | 132.20 [42.11, 132.20] | 4,208.50 | 1.20 | 0.11 |
Data are presented as median [interquartile range]. *, P<0.05; **, P<0.01. CB, chronic bronchitis; CRP, C-reactive protein; CT, computed tomography; Hb, hemoglobin; IL-6, interleukin-6; PLT, platelet; RBC, red blood cell count; WBC, white blood cell count.
Comparison of CT and mucus scores between initial and follow-up scans
In both group 1 and group 2, the initial and follow-up CT scores were lower in the non-CB group as compared to those in the CB group; however, this difference was not statistically significant. Conversely, the CT scores in the CB group were consistently higher at both initial and follow-up assessments, with no significant difference observed between these two time points.
Comparing the initial mucus scores between patients with CB and the non-CB patients in group 1, the scores ranged from 4.75 to 7.00, and this variability was not statistically significant (P=0.94). However, the follow-up results indicated a decrease in mucus scores for both groups, with a more pronounced reduction observed in patients without CB. The median score decreased to 2.5 in these patients as compared to 4.0 in those with CB. In group 2, the mucus scores of patients without CB remained consistently low before and after follow-up, and statistical tests revealed no significant difference between the two scores (P=0.36–0.20). Conversely, while the mucus scores of patients with CB were slightly higher than those without CB at the initial evaluation, they demonstrated a clear decrease at follow-up, although this reduction was not statistically significant (P=0.20) (Table 7).
Table 7
| Characteristics | Non-CB patient | CB patient | Z value | P value |
|---|---|---|---|---|
| CT score | ||||
| G1 | ||||
| Initial | 5.0 [0.0, 8.0] | 6.0 [1.5, 10.0] | −1.66 | 0.09 |
| Follow-up | 3.0 [0.0, 7.0] | 6.0 [0.0, 9.0] | −1.05 | 0.28 |
| G2 | ||||
| Initial | 10.0 [6.5, 12.5] | 8.0 [7.0, 9.5] | 1.10 | 0.27 |
| Follow-up | 5.0 [0.0, 13.0] | 7.0 [0.0, 9.0] | 0.58 | 0.56 |
| Mucus score | ||||
| G1 | ||||
| Initial | 6.00 [4.75, 7.00] | 6.00 [4.75, 7.00] | −0.09 | 0.94 |
| Follow-up | 2.50 [1.50, 5.00] | 4.00 [2.00, 5.25] | −0.80 | 0.43 |
| G2 | ||||
| Initial | 1.50 [0.50, 2.00] | 2.00 [0.50, 2.50] | −0.92 | 0.36 |
| Follow-up | 1.50 [0.50, 2.00] | 1.50 [0.50, 3.00] | −1.27 | 0.20 |
Data are presented as median [interquartile range]. G1: group 1, high mucus group; score ≥4. G2: group 2, low mucus group; score <4. CB, chronic bronchitis; CT, computed tomography.
Discussion
Respiratory viral infections trigger a cascade of pathological events initiated by neutrophil infiltration and subsequent epidermal growth factor receptor (EGFR)-mediated mucin biosynthesis, particularly MUC5AC overproduction (10). Concurrently, the expansion of antigen-presenting cells (monocytes, macrophages, and dendritic cells) and their excessive release of proinflammatory cytokines like IL-6 may precipitate systemic cytokine storm syndrome (11). Crucially, diverse respiratory viruses—including coronaviruses, influenza viruses, adenovirus (AdV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV)—share common pathogenic mechanisms involving both disruption of airway epithelial tight junctions and dysregulation of mucin expression profiles (12). These interconnected processes ultimately compromise mucociliary clearance capacity and establish a self-perpetuating cycle of mucus hypersecretion, mechanical airway obstruction, progressive ventilation impairment, and exacerbated inflammatory responses. The study (13) found that patients with CB were more likely to exhibit mucus hypersecretion than those without CB, a finding that aligns with the core features of CB pathophysiology. The research by scholars such as Angelis et al. has confirmed that the core pathophysiology of CB is chronic airway inflammation, primarily manifested as excessive mucus secretion, narrowing of small airways, and the development of emphysema, which further supports the findings of this study (14). Additionally, the mean age of patients with CB was significantly higher than that of those without CB, which is in line with epidemiological statistics (15,16) and suggests that CB primarily affects individuals over 40 years of age. This further strengthens the reliability and generalizability of the study’s conclusions.
Moreover, the study by Rathnayake et al. (17) found that smoking alters the cellular composition of the bronchial mucus barrier, reprograms the transcriptome, and increases mucus production. This is reflected in our data, which showed that the proportion of current smokers was significantly higher in the CB group than in the non-CB group. We hypothesize that this phenomenon may be associated with smoking-induced pathophysiological changes, such as activation of the EGFR/IL-13 signaling pathway by tobacco smoke, leading to goblet cell metaplasia and reduced ciliated cells (18). Upregulation of mucin-encoding genes (e.g., MUC5AC, MUC5B) further promotes mucus hypersecretion (19). These alterations collectively increase mucus accumulation in the airways, contributing to a cascade of clinical manifestations. The accumulation of airway mucus enhances susceptibility to viral infections while simultaneously impairing mucociliary clearance, resulting in aggravated infection and prolonged clinical course.
Widysanto et al. (5) reported that patients with CB experience long-term airway inflammation, leading to pathological changes such as wall thickening and small airway obstruction. These changes heighten the sensitivity of the airways to external stimuli such as viruses, thereby increasing the susceptibility of these patients to infection. Upon reinfection, these patients are more likely to experience alveolar mucosal damage and intense inflammatory responses. This further exacerbates mucus secretion and impairs gas exchange, ultimately resulting in hypoxia (20). Under hypoxic conditions, the body may have an elevated RBC count and Hb level through compensatory mechanisms, such as negative feedback regulation, to enhance the blood’s oxygen-carrying capacity. Consequently, it is hypothesized that RBC count and Hb level in patients with CB are higher than those in patients without CB. However, the findings of this study diverge from this expectation. A potential explanation for this discrepancy could be that prolonged hypoxia and compensatory pathological processes may affect the hematopoietic system or RBC function in patients with CB, resulting in relatively low RBC and Hb levels despite the hypoxic state (20).
Our study identified a noteworthy phenomenon in group 1: non-CB patients demonstrated significantly higher CRP levels compared to CB patients. This observation likely reflects the unique pathophysiology of CB progression, suggesting that CB patients exhibit distinct inflammatory response patterns differing from acute inflammation characteristics (12). Specifically, the long-term chronic airway inflammation in CB patients may lead to altered immunomodulatory mechanisms, manifesting as relative suppression of acute-phase protein release (including CRP) (21). Conversely, elevated CRP levels in non-CB patients may indicate recent acute infections or other unidentified proinflammatory states. This discovery challenges the conventional assumption that CB is invariably associated with more pronounced systemic inflammatory responses, highlighting the need for comprehensive evaluation of inflammatory profiles across patient subtypes. Critical considerations should include disease staging, comorbidities, and treatment history as potential confounding factors. Radiological research (5) has identified the imaging characteristics of patients with CB, such as GGO and bronchial vessel wall thickening. These findings align with the statistically significant features identified in our study. It is well-established that airway epithelial cell dysfunction due to CB can result in obstruction, contraction, and spasms of the small bronchi, which may progress to obstructive emphysema—a condition more common in patients with CB. Tana et al. (22) compared the bronchial lumen volumes between two patient groups and found that there were abnormal epithelial cell proliferation and invasive changes in the airway wall during the pathological CB process. This led to a reduced lumen diameter and obstructed airflow, resulting in a notably smaller lumen volume in patients with CB as compared to those without CB. Moreover, a comprehensive review of multiple studies (23) highlighted that excessive mucus secretion in the airways of patients with CB is a persistent issue, even after viral infection. In our study, we found that while mucus scores were decreased for both patient groups upon re-examination, the decline was more pronounced in patients without CB. The initial mucus scores for patients with CB were marginally higher than those without CB, consistent with the observation of heightened airway mucus secretion in these patients (24). This phenomenon not only worsens airway obstruction but also amplifies local hypoxia, creating an environment conducive to microbial growth and reproduction, thereby elevating infection risk (24). We found that both the initial and follow-up CT scores of CB patients were significantly higher than those without CB. It is speculated that this may have affected pneumonia absorption and daily activity capabilities in patients with CB (25).
Our study involved certain limitations which should be addressed. First, the limited sample size might reduce the representativeness of the sample, thereby making it challenging to generalize the conclusions to a wider patient population. Additionally, relevant confounding factors such as environmental pollution could not be incorporated into the study. Future research should include a larger sample size and an extended follow-up period to produce more robust findings. We have incorporated smoking status data, but the lack of detailed environmental exposure metrics (e.g., air pollution levels, occupational exposures) represents an important study limitation that should be addressed in future research. Second, the existing mucus scoring system only allows for a binary assessment of mucus severity at the lung segment level (26), which does not quantify the amount of mucus partially obstructing the airway lumen. Despite not causing complete airway closure, a partially obstructing mucus could significantly impact gas exchange, airway defense mechanisms, and microbial colonization. Meanwhile, there is a certain degree of unreliability in manually scoring mucus. Therefore, to enhance the accuracy and clinical utility for future studies, new methods or technologies should be explored to quantify mucus in the bronchial and lung segments. Third, consolidation areas resulting from lung infections could obscure the presence of mucus plugs, making their imaging manifestations difficult to identify. Morphological changes in the lung tissue due to consolidation could also alter the natural distribution pattern of mucus in the lungs, leading to inaccurate assessment results from the mucus scoring system. This confounding factor should thus be identified and controlled during data analysis and result interpretation, and experimental designs should aim to minimize interference. Finally, as we employed a single-center, retrospective design, the generalizability and reliability of the conclusions may be limited. To enhance the robustness and generalizability of the conclusions, multicenter, prospective studies should be conducted to further validate and consolidate the existing findings, thereby reducing the susceptibility to bias and errors inherent in single-center studies.
This study examined the variations in mucus secretion and inflammation levels in the lungs of patients with and without CB in the context of viral lung infections. The findings indicated that patients with CB were more susceptible to mucus hypersecretion following a viral infection, aligning with the primary pathological-physiological characteristics of persistent mucus hypersecretion. Furthermore, patients with CB tended to be older, which aligns with the epidemiological data. A comprehensive analysis of the pathophysiological mechanisms revealed that the airways of patients with CB were chronically inflamed, resulting in wall thickening, small airway obstruction, and increased sensitivity to viral stimuli. These factors intensify alveolar mucosal damage, inflammatory responses, and mucus secretion, ultimately impacting gas exchange and hypoxia. Moreover, we systematically analyzed the radiological manifestations in the lungs after viral infection, confirming the presence of specific changes such as emphysema and bronchial wall thickening in patients with CB. Following viral lung infections, the CT scores of patients with CB remained relatively high. Under conditions of high mucus secretion, the reduction in mucus score in patients with CB was less significant than that in patients without CB, suggesting that high mucus secretion adversely affects disease progression and prognosis.
Furthermore, Dal Negro et al.’s study (27) has indicated that for patients with CB who have a mucus plugging, the rehydration and restoration of mucous osmotic and viscous/elastic properties should be administered. Only when complementary hydrating and mucolytic agents are applied to clear the mucus accumulated in the lungs will airway obstruction, inflammation, and indeed, infection broadly improve (28).
In conclusion, this study examined the characteristics of CB in the context of mucus hypersecretion, emphysema, and bronchial wall thickening and clarified the distinct manifestations of CB within the framework of viral lung infections. Moreover, we identified the critical factors that increase the severity of viral pneumonia and shape its clinical outcomes, thereby offering novel insights and evidence for the diagnosis and management of CB.
Conclusions
The pathological features of CB, such as mucus hypersecretion, emphysema, and wall thickening, are key factors exacerbating the severity of viral pneumonia and worsening clinical outcomes. CB-specific pathological changes play a critical role in aggravating viral pneumonia through mechanisms like increased airway obstruction, localized hypoxia, and susceptibility to microbial proliferation. Understanding these associations emphasizes the importance of targeted therapeutic strategies, including improving mucus clearance, reducing inflammation, and mitigating structural damage, thereby enhancing prognosis and clinical outcomes in CB patients.
Acknowledgments
We sincerely thank Liyuan Han and Jiazhen Zhu for their contributions to the writing and polishing of this paper. The support from Ningbo No. 2 Hospital, including the provision of subjects and technical assistance, was crucial for the completion of this work. Finally, we would like to express our gratitude to all the participants in our study for their time and willingness to share their experiences.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-638/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-638/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-638/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-638/coif). All authors report that this work was supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LTGY23H180003 and LTGY23H010001), the Zhejiang Provincial Traditional Chinese Medical Scientific Research Foundation of China (Nos. 2024ZL929 and 2024ZL936), the Ningbo Natural Science Foundation of China (No. 2023J315), the Ningbo Clinical Research Center for Medical Imaging of China (No. 2021L003), and the Ningbo Leading Medical & Health Discipline of China (No. 2022-S02). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study received approval from the Ethics Committee for Human Research at Ningbo No. 2 Hospital (Ningbo, Zhejiang, China; approval No. YJ-NBEY-KY-2024-046-01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li Y, Tang XX. Abnormal Airway Mucus Secretion Induced by Virus Infection. Front Immunol 2021;12:701443. [Crossref] [PubMed]
- Kumar SS, Binu A, Devan AR, et al. Mucus targeting as a plausible approach to improve lung function in COVID-19 patients. Med Hypotheses 2021;156:110680. [Crossref] [PubMed]
- Respiratory Equipment Committee of China Association of Medical Equipment. Chinese experts consensus on pulmonary rehabilitation therapy for airway mucus hypersecretion. International Journal of Respiration 2021;41:1686-96.
- Jarhyan P, Hutchinson A, Khaw D, et al. Prevalence of chronic obstructive pulmonary disease and chronic bronchitis in eight countries: a systematic review and meta-analysis. Bull World Health Organ 2022;100:216-30. [Crossref] [PubMed]
- Widysanto A, Goldin J, Mathew G. Chronic Bronchitis. Treasure Island, FL, USA: StatPearls Publishing; 2025.
- Lim JU, Lee JH, Kim TH, et al. Alternative definitions of chronic bronchitis and their correlation with CT parameters. Int J Chron Obstruct Pulmon Dis 2018;13:1893-9. [Crossref] [PubMed]
- Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest 2018;128:997-1009. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Han X, Fan Y, Alwalid O, et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021;299:E177-86. [Crossref] [PubMed]
- Khorasani AM, Mohammadi B, Saghafi MR, et al. The association between MUC5AC and MUC5B genes expression and remodeling progression in severe neutrophilic asthma: A direct relationship. Respir Med 2023;213:107260. [Crossref] [PubMed]
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473-4. [Crossref] [PubMed]
- Zanin M, Baviskar P, Webster R, et al. The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016;19:159-68. [Crossref] [PubMed]
- Fortin M, Dorscheid DR, Liberman M, et al. Bronchial Rheoplasty for Chronic Bronchitis: Results from a Canadian Feasibility Study with RheOx Int J Chron Obstruct Pulmon Dis 2024;19:1673-80. [Crossref] [PubMed]
- Angelis N, Porpodis K, Zarogoulidis P, et al. Airway inflammation in chronic obstructive pulmonary disease. J Thorac Dis 2014;6:S167-72. [Crossref] [PubMed]
- Agarwal AK, Raja A, Brown BD. Chronic Obstructive Pulmonary Disease. Treasure Island, FL, USA: StatPearls Publishing; 2024.
- Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022;10:447-58. [Crossref] [PubMed]
- Rathnayake SNH, Ditz B, van Nijnatten J, et al. Smoking induces shifts in cellular composition and transcriptome within the bronchial mucus barrier. Respirology 2023;28:132-42. [Crossref] [PubMed]
- Czekala L, Wieczorek R, Simms L, et al. Multi-endpoint analysis of human 3D airway epithelium following repeated exposure to whole electronic vapor product aerosol or cigarette smoke. Curr Res Toxicol 2021;2:99-115. [Crossref] [PubMed]
- Radicioni G, Ceppe A, Ford AA, et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med 2021;9:1241-54. [Crossref] [PubMed]
- Febbo J, Revels J, Ketai L. Viral Pneumonias. Radiol Clin North Am 2022;60:383-97. [Crossref] [PubMed]
- Dahl M, Vestbo J, Lange P, et al. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:250-5. [Crossref] [PubMed]
- Tana A, Zhang C, DiBardino D, et al. Bronchoscopic interventions for chronic bronchitis. Curr Opin Pulm Med 2024;30:68-74. [Crossref] [PubMed]
- Sciurba FC, Dransfield MT, Kim V, et al. Bronchial rheoplasty for chronic bronchitis: 2-year results from a US feasibility study with RheOx. BMJ Open Respir Res 2023;10:e001710. [Crossref] [PubMed]
- Diaz AA, Orejas JL, Grumley S, et al. Airway-Occluding Mucus Plugs and Mortality in Patients With Chronic Obstructive Pulmonary Disease. JAMA 2023;329:1832-9. [Crossref] [PubMed]
- Minet E, Haswell LE, Corke S, et al. Application of text mining to develop AOP-based mucus hypersecretion genesets and confirmation with in vitro and clinical samples. Sci Rep 2021;11:6091. [Crossref] [PubMed]
- Mummy DG, Dunican EM, Carey KJ, et al. Mucus Plugs in Asthma at CT Associated with Regional Ventilation Defects at (3)He MRI. Radiology 2022;303:184-90. [Crossref] [PubMed]
- Dal Negro R, Pozzi E, Cella SG. Erdosteine: Drug exhibiting polypharmacy for the treatment of respiratory diseases. Pulm Pharmacol Ther 2018;53:80-5. [Crossref] [PubMed]
- Hill DB, Button B, Rubinstein M, et al. Physiology and pathophysiology of human airway mucus. Physiol Rev 2022;102:1757-836. [Crossref] [PubMed]
(English Language Editor: J. Gray)



