
Computed tomography-based body composition and systemic inflammation for outcome prediction in patients with resectable esophageal squamous cell carcinoma
Highlight box
Key findings
• Sarcopenia in esophageal squamous cell carcinoma (ESCC) was negatively associated overall survival (OS). The combination of sarcopenia, pathological parameters, and inflammatory factors could independently predict the survival of patients undergoing ESCC surgery, demonstrating good prognostic value.
What is known and what is new?
• Previous studies have shown that sarcopenia and inflammatory factors are associated with a poorer OS rate in patients with esophageal cancer after surgery.
• There has been no research on the combined use of sarcopenia and lymphocyte albumin score to evaluate the prognosis of patients after esophageal cancer surgery.
What is the implication, and what should change now?
• Sarcopenia was associated with a relatively low survival rate following ESCC surgery. A nomogram developed based on sarcopenia and clinical pathological data can be expected to predict the survival rate of patients after ESCC surgery and guide clinical treatment plans.
Introduction
Esophageal cancer (EC) stands as the seventh most common and sixth deadliest cancer globally (1). Esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC) represent the two primary histological subtypes of EC. Esophageal adenocarcinoma predominantly occurs in Western countries, whereas ESCC is more prevalent in East Asia, particularly in China and Japan (2). Diagnosis often reveals locally advanced, unresectable, or metastatic diseases, with a 5-year survival rate of 15–40%, even among patients undergoing esophagectomy (3).
Therefore, there is a pressing need in clinical practice to identify simple, cost-effective biomarkers capable of predicting ESCC prognosis, thereby aiding in survival assessment. Changes in body composition, which can include adipose tissue, skeletal muscle, and bone density, during tumor development can influence the prognosis of patients with cancer (4,5). Computed tomography (CT) scans, rich in information regarding bones, fat, and muscles, have been independently employed to assess disease status and prognosis (6). The European Working Group on Sarcopenia in Older People defines sarcopenia as a decrease in muscle mass, composition, mass, or strength (7). Sarcopenia, a skeletal muscle disease linked to chronic consumptive conditions, is associated with an increased likelihood of adverse consequences such as falls, fractures, physical disabilities, and death (8). It is also linked to unfavorable outcomes, increased recurrence risk, and metastasis in various cancer types, including EC (9-12). Thus, objectively measurable indicators of frailty or physical condition, such as sarcopenia and skeletal muscle mass, can enhance prognosis assessment and treatment stratification in patients with EC.
Systemic inflammation, a crucial characteristic of the tumor microenvironment, figures prominently in cancer progression and the prognosis of patients with cancer. Hematological inflammatory markers such as neutrophils, lymphocytes, platelets, and C-reactive protein effectively mirror the systemic inflammatory status in cancer (13,14). Several systemic inflammatory biomarkers comprising these parameters have been assessed, and their prognostic value in various cancers, including EC, has been confirmed (15-17). An association between sarcopenia and poorer survival outcomes in patients with EC, particularly when coupled with increased inflammation, has been demonstrated (18). These findings suggest that the coexistence of sarcopenia and inflammatory markers strongly reflects tumor invasiveness, rendering this combination a valuable predictor of long-term prognosis in patients with EC. We conducted a study to establish a survival model after esophagectomy, amalgamating a comprehensive set of body composition features from preoperative CT scans and clinical features, pathology and preoperative inflammatory factors. The study objectives were as follows: (I) to analyze the potential factors influencing patient survival through univariate and multivariate analyses and (II) to construct a nomogram for predicting survival after esophagectomy. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-508/rc).
Methods
Patient cohort
We collected data from 142 patients who underwent surgery for EC at The First Affiliated Hospital of Anhui Medical University between January 12, 2012, and January 12, 2021. The final follow-up date was January 12, 2023. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study received approval from the Ethics Committee of The First Affiliated Hospital of Anhui Medical University (approval No. quick-PJ 2024-03-22), and given its retrospective nature, written patient consent was not required.
The inclusion criteria were as follows: (I) histopathologically confirmed diagnosis of squamous cell carcinoma and R0 resection (complete microscopic resection with no residual tumor). All patients were staged following the eighth edition of the tumor-node-metastasis (TNM) stage staging system of the American Joint Committee on Cancer for EC; (II) preoperative CT scan of the chest and abdomen, including the third lumbar level; (III) no preoperative radiotherapy or chemotherapy; and (IV) availability of complete clinical data.
The exclusion criteria were as follows: (I) incomplete or poor-quality CT imaging data; (II) nonprimary EC or a history of other malignant tumors; and (III) presence of endocrine system or metabolic system diseases, such as diabetes and obesity, that could interfere with the analysis of human body composition.
Sarcopenia measurements and image analysis
All patients underwent CT examinations within two weeks before the surgery. Two doctors, each with over 10 years of experience in target area delineation, performed the segmentation of the region of interest. The third lumbar spine plane on abdominal CT was outlined using 3D Slicer software version 5.2.2. Standard CT values ranged from −29 to 150 Hounsfield units (HU) for muscle, −150 to −50 HU for visceral fat tissue, and −190 to +30 HU for subcutaneous fat tissue.
The parameters measured and how they were measured are as follows: (I) the skeletal muscle index was involved dividing the muscle area (cm2) by the subject’s height. Sarcopenia thresholds, based on previous research, were set at 34.4 cm2/m2 and 45.4 cm2/m2 for women and men, respectively (17). The original data were obtained from the third lumbar plane of CT images. (II) Body mass index was computed as the ratio of the square of weight (kg) to height (m). (III) Total abdominal skeletal muscle area (SMA) was defined as the sum of the total abdominal muscle area and visceral muscle area (mm2). (IV) Visceral fat area was calculated as the difference between total fat area and subcutaneous fat area (cm2). (V) Skeletal muscle density was defined as the average CT value (HU) within the cutting area corresponding to the SMA. (VI) The fat:muscle ratio was calculated as the ratio of total abdominal fat area to the total SMA in the abdomen. (VII) The visceral subcutaneous fat ratio was the ratio of visceral fat area in the abdomen to subcutaneous fat area in the abdomen. (VIII) The skeletal muscle:visceral fat ratio (SVR) was calculated as the ratio of the total abdominal SMA to abdominal visceral fat area. (IX) The visceral fat index and subcutaneous fat index were calculated by dividing the corresponding fat area by the square of the patient’s height.
Study criteria
Preoperative blood test data were obtained from the cases. The systemic immune-inflammation index is defined as platelet (/µL) × neutrophil (/µL)/lymphocyte (/µL), neutrophil-platelet score is defined as neutrophil (/µL) × platelet score (/µL), and lymphocyte-albumin score is defined as lymphocyte (/µL) × albumin (g/dL) score. The systemic inflammation score (SIS), based on serum albumin and lymphocyte:monocyte ratio (LMR), is as a novel prognostic tool for some tumors. The SIS comprises serum albumin levels and LMR, and stratified patients in this study as follows: patients with albumin level >4.0 g/dL and LMR >4.44 were scored 0, patients with albumin level <4.0 g/dL and LMR <4.44 was scored 2, and other patients were scored 1.
Follow-up and outcomes
Regular visits and telephone follow-ups were conducted at the outpatient or inpatient department of The First Affiliated Hospital of Anhui Medical University.
Within 1–2 years following treatment, follow-ups were scheduled every 3–6 months, every 6 months for 3–5 years, and once a year after 5 years. The primary endpoint of this study was overall survival (OS), defined as the time from ESCC surgery to death from any cause.
Statistical analysis
All analyses were conducted using R version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS 28.0 (IBM Corp., Armonk, NY, USA). Based on the cutoff values assessed by X-tile 3.6.1 software (Yale University School of Medicine, New Haven, CT, USA), all numerical variables were converted into categorical variables for application. Continuous variables conforming to normal distribution are expressed as the mean ± standard deviation, those with a skewed distribution are expressed as the median, and categorical variables are expressed as the frequency and percentage. The Kaplan-Meier method calculated the survival curve, and log-rank tests were used to test differences between survival curves. Spearman or Pearson correlation analysis was used to determine the correlation coefficient between each characteristic. Pearson correlation analysis was employed when normal distribution was satisfied, and Spearman correlation analysis was used when normal distribution was not satisfied. For a set of features with a correlation coefficient greater than 0.9, one of them was randomly removed. Univariate and multivariate Cox regression analyses screened variables associated with long-term survival, and the nomogram model was constructed via Cox regression. The concordance index (C-index) and calibration curve were selected to evaluate the prediction accuracy of the nomogram. A significance level of P<0.05 indicated statistical significance.
Results
Demographic and clinicopathological features of patients with ESCC
A total of 142 patients were included in this study cohort, all diagnosed with ESCC (Figure 1). The median follow-up was 45.0 [95% confidence interval (CI): 39.0–55.0] months. At baseline, 88 patients were diagnosed with sarcopenia, accounting for 62.0% of the cohort. Table 1 outlines the baseline clinical characteristics, indicating an average age of 65.2 (range, 39–82) years and a predominantly male population (116/142, 81.7%). Stage I patients constituted 18.3% (26/142) of the cohort, while stage II patients accounted for 56.3% (80/142). The most common clinical T stages were T1–2 (87/142, 61.3%), with the most prevalent clinical N stage being N0 (105/142, 73.9%).
Table 1
| Characteristic | Value |
|---|---|
| 1. Age (years) | 65.2±8.0 |
| <65 | 64 |
| ≥65 | 78 |
| 2. Weight (kg) | 60.2±10.0 |
| ≤60 | 77 |
| >60 | 65 |
| 3. Male sex | 116 |
| 4. Smoking | 72 |
| 5. Alcohol | 55 |
| 6. Tumor location | |
| Upper | 9 |
| Middle | 86 |
| Lower | 47 |
| 7. Tumor grade | |
| Well-differentiated | 46 |
| Moderately-differentiated | 82 |
| Poorly-differentiated | 14 |
| 8. T stage | |
| T1–T2 | 87 |
| T3–T4 | 55 |
| 9. N stage | |
| N0 | 105 |
| N1–3 | 37 |
| 10. TNM stage (8th edition, 2017) | |
| I–II | 106 |
| III–IV | 36 |
| 11. Tumor length (cm) | |
| <4 | 86 |
| ≥4 | 56 |
| 12. BMI (kg/m2) | |
| <18.5 | 22 |
| ≥18.5 | 120 |
| Body composition before surgery | |
| 13. Sarcopenia | 88 |
| 14. Total fat area (mm2)* | 14,298.7±8,951.5 |
| 15. Visceral fat area (mm2)* | 7,335.0±5,506.12 |
| 16. Visceral fat index (cm2/m2) | 26.5±19.6 |
| 17. Subcutaneous fat area (mm2)* | 6,963.7±4,290.3 |
| 18. Subcutaneous fat index (cm2/m2) | 25.5±16.6 |
| 19. Visceral fat:subcutaneous fat ratio | 1.1±0.6 |
| 20. Subcutaneous fat:muscle ratio* | 0.6±0.4 |
| 21. Height:waist ratio | 2.2±0.3 |
| Laboratory parameters | |
| 22. Platelet (109/L)* | 196.7±75.2 |
| 23. White blood cell (109/L) | 6.1±1.7 |
| 24. Neutrophil (109/L)* | 3.8±1.4 |
| 25. Lymphocyte (109/L) | 1.7±0.6 |
| 26. Monocytes (109/L)* | 0.4±0.1 |
| 27. Lymphocyte:monocyte ratio | 4.8±2.4 |
| 28. Neutrophil:lymphocyte ratio | 2.7±1.9 |
| 29. Platelet:albumin ratio | 4.7±1.8 |
| 30. Neutrophil:albumin ratio | 0.1±0.03 |
| 31. Platelet:lymphocyte ratio | 135.7±91.9 |
| 32. Systemic immune-inflammation index | 543.9±490.5 |
| 33. Neutrophil-platelet score | 794.5±546.4 |
| 34. Lymphocyte-albumin score | 16.5±6.0 |
| 35. Albumin (g/L) | 42.0±4.1 |
| 36. Lactate dehydrogenase (U/L) | 170.6±33.2 |
Data are presented as mean ± standard deviation or numbers. *, according to the correlation matrix, for a set of features with a correlation coefficient greater than 0.9, one of the features was randomly removed. BMI, body mass index; TNM, tumor-node-metastasis.
Survival outcomes and Cox regression analysis
During the follow-up period, 69 (69/142, 48.6%) patients died. The overall median OS for patients was 74.0 (95% CI: 48.8–99.2) months. For patients with sarcopenia, the median OS and 1, 3, and 5-year survival rates were 54.0 (95% CI: 37.8–70.2) months, 79.5%, 59.1%, and 44.5% respectively. Patients without sarcopenia had a median OS of 93.0 (95% CI: 70.0–116.0) months, and the 1-, 3-, and 5-year survival rates were 90.0%, 73.9%, and 65.1%, respectively (P=0.04 for the comparison of OS between the two groups; Figure 2).

According to the correlation matrix, for a set of features with a correlation coefficient greater than 0.9, one of the features was randomly removed (Figure 3). After randomly eliminating characteristics (total fat area, visceral fat area, subcutaneous fat area, subcutaneous fat:muscle ratio, platelet, neutrophils, and monocytes), the variables were selected according to clinical needs for univariate Cox regression analysis (Table 2). The factors that demonstrated a significant association with OS included smoking [hazard ratio (HR) =0.434, 95% CI: 0.264–0.715; P<0.001], TNM stage (HR =0.394, 95% CI: 0.241–0.644; P<0.001), tumor length (HR =0.616, 95% CI: 0.381–0.999; P=0.049), sarcopenia (HR =1.874, 95% CI 1.102–3.188; P=0.02), subcutaneous fat index (HR =1.482, 95% CI: 1.023–2.145; P=0.04), visceral fat:subcutaneous fat ratio (HR =0.384, 95% CI: 0.175–0.842; P=0.02), and lymphocyte-albumin score (HR =0.486, 95% CI: 0.294–0.805; P=0.004).
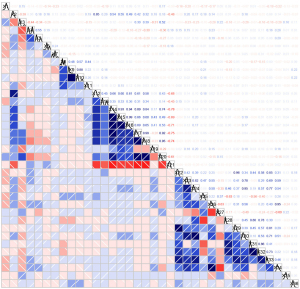
Table 2
| Characteristic | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (<65 vs. ≥65 years) | 1.278 | 0.795–2.056 | 0.31 | ||||
| Sex (male vs. female) | 0.655 | 0.335–1.282 | 0.22 | ||||
| Smoking (no vs. yes) | 0.434 | 0.264–0.715 | <0.001 | 0.457 | 0.263–0.792 | 0.005 | |
| Tumor location (upper or middle vs. lower) | 0.800 | 0.475–1.349 | 0.40 | ||||
| TNM stage (I+II vs. III+IV) | 0.394 | 0.241–0.644 | <0.001 | 0.368 | 0.220–0.615 | <0.001 | |
| Tumor length (<4 vs. ≥4 cm) | 0.616 | 0.381–0.999 | 0.049 | 0.829 | 0.399–1.720 | 0.61 | |
| Sarcopenia (yes vs. no) | 1.874 | 1.102–3.188 | 0.02 | 1.685 | 1.002–2.832 | 0.049 | |
| Visceral fat index (<27 vs. ≥27 cm2/m2) | 1.392 | 0.856–2.266 | 0.18 | ||||
| Subcutaneous fat index (<41.8 vs. ≥41.8 cm2/m2) | 1.482 | 1.023–2.145 | 0.04 | 1.361 | 0.574–2.225 | 0.48 | |
| Visceral fat:subcutaneous fat ratio (<0.6 vs. ≥0.6) | 0.384 | 0.175–0.842 | 0.02 | 0.762 | 0.342–1.697 | 0.51 | |
| Lymphocyte:monocyte ratio (<4.5 vs. ≥4.5) | 0.954 | 0.857–1.061 | 0.38 | ||||
| Neutrophil:lymphocyte ratio (<1.4 vs. ≥1.4) | 1.167 | 0.853–1.597 | 0.33 | ||||
| Lymphocyte-albumin score (<14.6 vs. ≥14.6) | 0.486 | 0.294–0.805 | 0.004 | 0.568 | 0.336–0.962 | 0.04 | |
OS, overall survival; EC, esophageal cancer; HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis.
In the multivariate model, the factors that remained associated with OS included TNM stage (HR =0.368, 95% CI: 0.220–0.615; P<0.001), sarcopenia (HR =1.685, 95% CI: 1.002–2.832; P=0.049), smoking (HR =0.457, 95% CI: 0.263–0.792; P=0.005), and lymphocyte-albumin score (HR =0.568, 95% CI: 0.336–0.962, P=0.04). The Kaplan-Meier method indicated significant differences in the survival curves of risk factors including smoking, TNM stage, sarcopenia, and lymphocyte-albumin score (Figure 2).
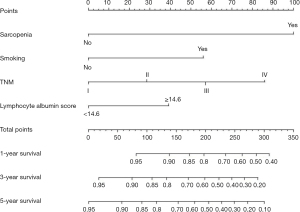
Nomogram development and validation
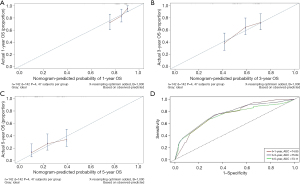
In the multivariate survival analysis, smoking, TNM stage, sarcopenia, and lymphocyte-albumin score emerged as independent factors for OS in patients with ESCC. The OS nomogram indicated that smoking, sarcopenia, late stage, and lymphocyte albumin score had higher predictive scores, correlating with a higher incidence of adverse OS (Figure 4). TNM was identified as the factor most associated with the prognosis of patients with postoperative ESCC, followed by smoking. The nomogram was validated, revealing a C-index of 0.71. The area under the curve values at 1, 3, and 5 years for the receiver operating characteristic were 74.85, 75.84, and 73.11, respectively (Figure 5). Furthermore, the calibration curves of the OS nomogram at 1, 3, and 5 years demonstrated alignment between the predicted survival probability and the actual observed values (Figure 5A-5C).
Discussion
TNM staging remains a vital tool for assessing the prognosis of patients with ESCC; however, variability in prognoses exist among patients with the same pathological stage. Integrating preoperative sarcopenia, clinicopathological data, and inflammatory factors may enhance the identification of patient risk and subsequently improve short- and long-term survival. Although single muscle measurement of the psoas major muscle at the third lumbar (L3 vertebra) has been considered a substitute for detecting sarcopenia, its reliability and accuracy require confirmation. Studies, such as that by Rutten et al., which included 150 patients with ovarian cancer, have reported poor correlation and kappa agreement between total lumbar area and psoas area (19). This discrepancy is attributed to the psoas muscle’s susceptibility to degenerative lumbar diseases, making it less specific to patients with cancer. Additionally, the irregular and small size of the psoas muscle in relation to the total L3 muscle tissue can lead to high measurement errors. Some studies have not found there to be a correlation between sarcopenia and shorter OS in patients with cancer (20-24); however, the majority of research indicates that sarcopenia is associated with a worse cancer prognosis (25-28).
The third lumbar spine (L3) segment is considered the main site for CT muscle mass measurement, encompassing all relevant muscles (including the psoas major, erector spinae, transverse abdominis, rectus abdominis, quadratus lumborum, and obliques) (29). The skeletal muscle index calculated through the L3 segment has demonstrated a strong correlation with overall muscle mass, making it a reliable method for sarcopenia evaluation (29). In the context of EC, a study has reported a sarcopenia incidence of up to 75%, highlighting the need to assess its prognostic value for patients with EC (30). Watanabe et al. retrospectively analyzed 135 patients who underwent esophagectomy, with 35 being diagnosed with sarcopenia. The overall 5-year survival rates for patients with and without sarcopenia were 28.9% and 58.9%, respectively. The 5-year disease-free survival rate for patients with sarcopenia was 34.1%, whereas that for patients without sarcopenia was 62.8% (31). Tamandl et al. reported a worse median OS for patients with preoperative EC than for patients without sarcopenia (31.5 vs. 76.5 months) (32). In our study, the median OS and 1-, 3-, and 5-year survival rates for patients with sarcopenia and ESCC were 54.0 months, 79.5%, 59.1%, and 44.5%, respectively. Meanwhile, the median OS and 1-, 3-, and 5-year survival rates for patients without sarcopenia were 93.0 months, 90.0%, 73.9%, and 65.1%, respectively. Thus, sarcopenia in patients with ESCC may be a promising prognostic factor, with the median OS and survival rates indicating its significant impact.
Systemic inflammation serves as a hallmark of cancer and is often triggered by oxygen deprivation or necrosis within tumor tissue. This disruption leads to an imbalance between inflammatory cells (neutrophils and monocytes) and tumor-specific lymphocytes (33). Zhang et al. established that a high preoperative systemic immune-inflammation index and low prognostic nutritional index are powerful indicators of invasive biology and poor prognosis in patients with ESCC (34). Another study by Huang et al. involving 166 older adult patients with ESCC undergoing radiotherapy found associations of decreased albumin level and LMR with a shorter OS, while a lower SIS was significantly correlated with better outcomes (15). In our study, the lymphocyte-albumin score was associated with prognosis.
Nomograms are commonly employed in tumor diagnosis and prognosis prediction due to their high precision and strong discriminatory ability (35-37). In our study, a prognostic nomogram was constructed based on smoking, TNM stage, sarcopenia, and lymphocyte-albumin score to calculate the OS rate. TNM emerged as the most crucial factor affecting the prognosis of postoperative patients with ESCC, followed by smoking. The nomogram demonstrated effective performance in predicting OS, with a C-index of 0.64. Calibration curves for OS probabilities at 12-, 36-, and 60-month intervals after treatment illustrated excellent agreement between the nomogram’s predictions and actual observations. These findings suggest that our nomogram holds significant potential for broader application in clinical settings.
Nonetheless, there are several limitations to our study that need to be considered. First, there was a lack of a systematic assessment of patients’ nutritional status. Although SMA and body mass index were analyzed during inclusion, a more comprehensive evaluation of nutritional factors could provide a more nuanced understanding. Second, the sample size was small, emphasizing the need for validation in a larger cohort to enhance the generalizability of the results. Third, the basic strategy for patients with EC, such as adjuvant or neoadjuvant treatment, was unclear. During the long study period, the standard strategy might have changed substantially.
Conclusions
In conclusion, this retrospective study identified sarcopenia as a factor associated with lower survival rates following ESCC surgery. The development of a nomogram based on sarcopenia and clinical pathological data represents a significant contribution to ESCC research. Notably, the nomogram requires no special equipment, entails no radiation damage, and uses simple calculation. Its facile operability and wide application prospects position it as a noninvasive tool with the potential to predict the survival rate of patients with postoperative ESCC and guide clinical treatment plans.
Acknowledgments
We thank Dr. Giovanni Pirozzolo (Cittadella Hospital, Padua, Italy) for the critical comments and valuable advice on this study.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-508/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-508/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-508/prf
Funding: This paper was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-508/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was reviewed and approved by The First Affiliated Hospital of Anhui Medical University (approval No. quick-PJ 2024-03-22). As this study was retrospective, the need for the informed consent of patients was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu H, Ma X, Ye T, et al. Esophageal cancer in China: Practice and research in the new era. Int J Cancer 2023;152:1741-51. [Crossref] [PubMed]
- Li L, Jiang D, Zhang Q, et al. Integrative proteogenomic characterization of early esophageal cancer. Nat Commun 2023;14:1666. [Crossref] [PubMed]
- Anconina R, Ortega C, Metser U, et al. Combined 18 F-FDG PET/CT Radiomics and Sarcopenia Score in Predicting Relapse-Free Survival and Overall Survival in Patients With Esophagogastric Cancer. Clin Nucl Med 2022;47:684-91. [Crossref] [PubMed]
- Cohen B, Hiller N, Szalat A, et al. OPPORTUNISTIC EVALUATION OF BONE MINERAL DENSITY BY PET-CT IN HODGKIN LYMPHOMA PATIENTS. Endocr Pract 2019;25:869-76. [Crossref] [PubMed]
- Wang X, Zhang C, Cao F, et al. Nomogram of Combining CT-Based Body Composition Analyses and Prognostic Inflammation Score: Prediction of Survival in Advanced Epithelial Ovarian Cancer Patients. Acad Radiol 2022;29:1394-403. [Crossref] [PubMed]
- Iyer K, Beeche CA, Gezer NS, et al. CT-Derived Body Composition Is a Predictor of Survival after Esophagectomy. J Clin Med 2023;12:2106. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [Crossref] [PubMed]
- Li S, Xie K, Xiao X, et al. Correlation between sarcopenia and esophageal cancer: a narrative review. World J Surg Oncol 2024;22:27. [Crossref] [PubMed]
- Li ZZ, Yan XL, Jiang HJ, et al. Sarcopenia predicts postoperative complications and survival in colorectal cancer patients with GLIM-defined malnutrition: Analysis from a prospective cohort study. Eur J Surg Oncol 2024;50:107295. [Crossref] [PubMed]
- Xiao X, Fang PH, Zhou JF, et al. Impact of Skeletal Muscle Loss and Sarcopenia on Outcomes of Locally Advanced Esophageal Cancer during Neoadjuvant Chemoradiation. Ann Surg Oncol 2024;31:3819-29. [Crossref] [PubMed]
- Yin L, Song C, Cui J, et al. Association of possible sarcopenia with all-cause mortality in patients with solid cancer: A nationwide multicenter cohort study. J Nutr Health Aging 2024;28:100023. [Crossref] [PubMed]
- Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med 2020;18:360. [Crossref] [PubMed]
- Jaillon S, Ponzetta A, Di Mitri D, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 2020;20:485-503. [Crossref] [PubMed]
- Huang C, Wang M, Chen L, et al. The pretherapeutic systemic inflammation score is a prognostic predictor for elderly patients with oesophageal cancer: a case control study. BMC Cancer 2023;23:505. [Crossref] [PubMed]
- Qi WX, Wang X, Li C, et al. Pretreatment absolute lymphocyte count is an independent predictor for survival outcomes for esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiotherapy and pembrolizumab: An analysis from a prospective cohort. Thorac Cancer 2023;14:1556-66. [Crossref] [PubMed]
- Russo E, Guizzardi M, Canali L, et al. Preoperative systemic inflammatory markers as prognostic factors in differentiated thyroid cancer: a systematic review and meta-analysis. Rev Endocr Metab Disord 2023;24:1205-16. [Crossref] [PubMed]
- Sugawara K, Yagi K, Uemura Y, et al. Associations of Systemic Inflammation and Sarcopenia With Survival of Esophageal Carcinoma Patients. Ann Thorac Surg 2020;110:374-82. [Crossref] [PubMed]
- Rutten IJ, Ubachs J, Kruitwagen RF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol 2017;43:717-24. [Crossref] [PubMed]
- Herrmann T, Mione C, Montoriol PF, et al. Body Mass Index, Sarcopenia, and Their Variations in Predicting Outcomes for Patients Treated with Nivolumab for Metastatic Renal Cell Carcinoma. Oncology 2022;100:114-23. [Crossref] [PubMed]
- Yoon HG, Oh D, Ahn YC, et al. Prognostic Impact of Sarcopenia and Skeletal Muscle Loss During Neoadjuvant Chemoradiotherapy in Esophageal Cancer. Cancers (Basel) 2020;12:925. [Crossref] [PubMed]
- Rutten IJG, Ubachs J, Kruitwagen RFPM, et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630-8. [Crossref] [PubMed]
- Matsuoka H, Nakamura K, Matsubara Y, et al. Sarcopenia Is Not a Prognostic Factor of Outcome in Patients With Cervical Cancer Undergoing Concurrent Chemoradiotherapy or Radiotherapy. Anticancer Res 2019;39:933-9. [Crossref] [PubMed]
- Thormann M, Heitmann F, Wrobel V, et al. Sarcopenia does not limit overall survival in patients with colorectal liver metastases undergoing interstitial brachytherapy. Rofo 2023;195:217-23. [Crossref] [PubMed]
- Jogiat UM, Baracos V, Turner SR, et al. Changes in Sarcopenia Status Predict Survival Among Patients with Resectable Esophageal Cancer. Ann Surg Oncol 2023;30:7412-21. [Crossref] [PubMed]
- Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann Surg 2017;266:822-30. [Crossref] [PubMed]
- Yu JI, Choi C, Lee J, et al. Effect of baseline sarcopenia on adjuvant treatment for D2 dissected gastric cancer: Analysis of the ARTIST phase III trial. Radiother Oncol 2020;152:19-25. [Crossref] [PubMed]
- Hacker UT, Hasenclever D, Baber R, et al. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann Oncol 2022;33:685-92. [Crossref] [PubMed]
- Pigneur F, Di Palma M, Raynard B, et al. Psoas muscle index is not representative of skeletal muscle index for evaluating cancer sarcopenia. J Cachexia Sarcopenia Muscle 2023;14:1613-20. [Crossref] [PubMed]
- Fang P, Zhou J, Xiao X, et al. The prognostic value of sarcopenia in oesophageal cancer: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2023;14:3-16. [Crossref] [PubMed]
- Watanabe A, Oshikiri T, Sawada R, et al. Actual Sarcopenia Reflects Poor Prognosis in Patients with Esophageal Cancer. Ann Surg Oncol 2022;29:3670-81. [Crossref] [PubMed]
- Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 2016;26:1359-67. [Crossref] [PubMed]
- Liang H, Peng H, Chen L. Prognostic Value of Sarcopenia and Systemic Inflammation Markers in Patients Undergoing Definitive Radiotherapy for Esophageal Cancer. Cancer Manag Res 2021;13:181-92. [Crossref] [PubMed]
- Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol 2019;234:1794-802. [Crossref] [PubMed]
- Liu L, Xiao Y, Wei D, et al. Development and validation of a nomogram for predicting suicide risk and prognostic factors in bladder cancer patients following diagnosis: A population-based retrospective study. J Affect Disord 2024;347:124-33. [Crossref] [PubMed]
- Yan X, Fu X, Gui Y, et al. Development and validation of a nomogram model based on pretreatment ultrasound and contrast-enhanced ultrasound to predict the efficacy of neoadjuvant chemotherapy in patients with borderline resectable or locally advanced pancreatic cancer. Cancer Imaging 2024;24:13. [Crossref] [PubMed]
- Ren J, Yuan Y, Qi M, et al. MRI-based radiomics nomogram for distinguishing solitary fibrous tumor from schwannoma in the orbit: a two-center study. Eur Radiol 2024;34:560-8. [Crossref] [PubMed]



