
Phenotypes and endotypes in bronchiectasis: a narrative review of progress toward precision medicine
Introduction
Bronchiectasis, formerly regarded as a rare disease, has recently gained renewed interest, leading to significant advancements in its research, therapeutic development, and research investment (1,2). Disease heterogeneity is the most challenging aspect of bronchiectasis management. This variability arises naturally, as bronchiectasis can occur as a standalone suppurative pulmonary disease or, more commonly, as the final common pathway of various infectious, genetic, autoimmune, and allergic disorders (3-5). Furthermore, significant clinical heterogeneity exists even among patients with the same etiology. This heterogeneity further complicates the development of uniform management strategies.
How can we address the challenges associated with the highly heterogeneous nature of bronchiectasis? This review aimed to discuss the concepts of phenotype and endotype and the recent advancements in their application to bronchiectasis. These insights will aid in navigating the challenging heterogeneity of bronchiectasis and contribute to the development of personalized treatments. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1945/rc).
Methods
Source references were identified through a PubMed search of abstracts and articles published in English between January 1990 and August 2024. The following search terms were used either alone or in combination; “bronchiectasis”, “phenotype”, “endotype”, “diagnosis”, “disease management”, “complexity”, “eosino*”, “neutrop*”, and “precision medicine”. The reference lists of original articles, narrative reviews, clinical guidelines, previous systematic reviews, and meta-analyses were examined to find additional relevant materials. The citations of articles identified through these searches were also reviewed and included. This review did not cover cystic fibrosis and traction bronchiectasis due to interstitial lung disease. Both authors assessed the eligibility (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | The initial search was conducted on 3 September 2024 |
| Database searched | PubMed |
| Search terms used | Terms used included “bronchiectasis”, “phenotype”, “endotype”, “diagnosis”, “disease management”, “complexity”, “eosino*”, “neutrop*”, and “precision medicine” |
| Timeframe | Published between January 1990 and August 2024 |
| Inclusion criteria | English language abstracts and articles |
| Selection process | Both authors identified source references. Randomized trials, observational studies, translational studies, and systematic and narrative reviews were considered. The reference lists of the original articles, narrative reviews, clinical guidelines, previous systematic reviews and meta-analyses were manually searched to identify additional relevant material. The citation lists from articles identified in these searches were also reviewed and included, where appropriate |
Phenotypes and endotypes of bronchiectasis
Definition of phenotype and endotype
Phenotypes are defined as observable characteristics related to clinically meaningful outcomes (6,7). This review mainly focuses on clinical phenotypes. For example, if a subset of bronchiectasis patients with frequent exacerbations (an observable characteristic) exhibit higher long-term mortality rates (a clinically meaningful outcome) compared with those without frequent exacerbations, this group can be classified as the “frequent exacerbator” phenotype. Thus, phenotyping bronchiectasis helps clarify the clinical problem. However, a limitation of phenotyping is that identifying the phenotype alone does not provide a solution.
Endotypes are described as biological classifications based on specific pathophysiological processes or biomarkers, offering insights into the unresolved questions surrounding clinical phenotypes (8). For example, two patients with different etiologies, radiological extents, and microbiological test results experienced more than three episodes of exacerbations in the previous year. Although both patients can be identified as frequent exacerbator phenotypes, biomarker testing may further classify the endotypes. If the test results indicate that eosinophilic and neutrophilic inflammation are the underlying causes of frequent exacerbations in a particular patient, targeted treatments can be implemented. Inhaled corticosteroids (ICS) or monoclonal antibodies targeting the T-helper cell type 2 (Th2) pathways may be utilized for the treatment of eosinophilic inflammation (9,10), whereas macrolides or dipeptidyl peptidase 1 (DPP-1) inhibitors may be suitable for managing neutrophilic inflammation (11,12). Thus, identifying endotypes can precisely guide the management of treatable traits and ultimately determine the therapeutic strategies tailored to the unique pathophysiological mechanisms in patients with bronchiectasis. Notably, one phenotype may not be related to only one endotype but to multiple endotypes to aid in more effective problem-solving. Figure 1 depicts the concepts of phenotypes and endotypes.

Phenotypes in bronchiectasis
Etiology-associated phenotypes
The initial step in managing bronchiectasis is identifying the underlying cause. In some cases, the identified etiologies may serve as bronchiectasis phenotypes. Non-tuberculous mycobacterial pulmonary disease (NTM-PD), allergic bronchopulmonary aspergillosis (ABPA), and immunodeficiency are associated with targeted treatments (13-15). Meanwhile, rheumatoid arthritis, bronchiectasis-chronic obstructive pulmonary disease (COPD) overlap, and primary ciliary dyskinesia (PCD) are associated with worse outcomes or require adjustments in management (2,16-18).
However, etiology-based phenotyping presents challenges in clinical practice. The international registry data indicate that 29–41% of bronchiectasis cases are idiopathic (19-24). Additionally, post-infective bronchiectasis accounts for 20–27% of cases (19-24). The lack of specific therapies for post-infective bronchiectasis makes it clinically similar to idiopathic bronchiectasis from the clinicians’ perspectives. Therefore, approximately 70–80% of patients with bronchiectasis may gain limited benefits from etiological investigations. From a different point of view, the observed low adherence to guideline-recommended etiologic screening and incomplete diagnoses raises concerns about the reliability of these data (21,25). Genetic testing, which remains significantly underutilized, indicates that over 10% of patients may have PCD, providing potential insights into idiopathic or post-infective bronchiectasis (17) (discussed further below). Improving the accuracy of etiological diagnoses and conducting in-depth research on the pathogenesis of bronchiectasis could advance etiology-based management strategies and ultimately improve patient care and outcomes.
Non-tuberculous mycobacteria pulmonary disease phenotype
Among the etiology-associated phenotypes, we aimed to highlight NTM-PD. The association between bronchiectasis and NTM-PD is well-known (26-28), and approximately 13% of patients with bronchiectasis were diagnosed with NTM-PD in a Southern European bronchiectasis registry (29). Yang et al. used a Korean nationwide 10-year follow-up dataset and revealed that the incidence of NTM-PD in those with bronchiectasis was approximately 19-fold higher than that in patients without bronchiectasis, even after adjusting for various confounding factors (30).
What clinical features can aid in identifying NTM-PD in patients with bronchiectasis? NTM-PD is disproportionally more prevalent in women, and its incidence increases with age. However, whether the female sex or exposure to female hormones is a risk factor for NTM-PD development remains unclear (31-33). Additionally, the typical radiological features of NTM-PD include bronchiectasis, which is most prevalent in the right middle lobe or lingula; multifocal nodular or cavitary opacities; and multiple small nodules (3,34,35). Therefore, clinicians should recognize the high incidence of NTM-PD in patients with bronchiectasis and carefully assess clinical indicators in those with suspected cases (36), because a diagnosis of NTM-PD may necessitate antibiotic treatment and lead to significant changes in the clinical course.
What are the clinical outcomes in patients with bronchiectasis and an NTM phenotype? A recent study from the United States Bronchiectasis and NTM Research Registry compared the clinical outcomes between 1,549 patients (59%) with NTM and 1,085 patients (41%) without NTM at baseline (37). The NTM status was determined based on the responses to the questions in the case report form, which included details regarding the diagnosis of NTM, mycobacterial culture results confirming NTM, and treatments specific to NTM. Notably, outcomes, including exacerbations, hospitalizations, rate of lung function decline, and mortality rate, were comparable between the two groups during the 5-year follow-up (37). These results suggest that the NTM phenotype is not associated with poor outcomes in patients with bronchiectasis or that the outcomes may have been influenced by appropriate NTM-PD treatment.
P. aeruginosa phenotype
The most well-characterized bronchiectasis phenotype is chronic bronchial infection by Pseudomonas aeruginosa (hereafter referred to as chronic P. aeruginosa infection or P. aeruginosa phenotype), which is associated with reduced health-related quality of life, more frequent exacerbations, higher rates of hospital admissions, and increased mortality (38-46). Therefore, chronic P. aeruginosa infection has been the focus of numerous randomized controlled trials evaluating inhaled antibiotics for bronchiectasis (47), with both existing and ongoing studies investigating strategies for eradicating this bacterium (48,49). Furthermore, international bronchiectasis guidelines recommend management strategies based on the status of chronic P. aeruginosa infection (13,15), and clinical trials on bronchiectasis often stratify participants based on the presence or absence of chronic P. aeruginosa infection (12,50,51).
Interestingly, chronic P. aeruginosa infections affect patients with bronchiectasis differently. A European multicenter study reported a high mortality risk in patients with bronchiectasis with a combination of P. aeruginosa infection and two or more exacerbations per year. In contrast, those with P. aeruginosa infection without frequent exacerbations had a favorable prognosis (38). These results suggest that the combination of P. aeruginosa infection and frequent exacerbations is associated with worse outcomes.
Frequent exacerbator phenotype
The frequent exacerbator phenotype is also well-distinguished in patients with bronchiectasis, along with the P. aeruginosa phenotype. In a European multicenter cohort study (n=2,572), the baseline exacerbation frequency was categorized into zero, one, two, or three or more exacerbations per year, and long-term clinical outcomes were followed up for up to 5 years (52,53). Notably, a history of frequent exacerbations was the strongest predictor of future exacerbations, with the risk increasing in a dose-dependent manner according to the frequency of previous exacerbations. The incidence rate ratio for future exacerbation was 1.73 [95% confidence interval (CI): 1.47–2.02] for 1 exacerbation per year, 3.14 (95% CI: 2.70–3.66) for 2 exacerbations, and 5.97 (95% CI: 5.27–6.78) for three or more exacerbations (52). This phenotype was relatively stable over time, a pattern similar to that observed in COPD, wherein patients with frequent exacerbations are more susceptible to the subsequent exacerbations, independent of lung function (54).
As the frequent exacerbator phenotype is associated with increased hospitalization rates and higher 5-year mortality rates (52), the international bronchiectasis guidelines recommend management strategies aimed at preventing exacerbations (13,15). Furthermore, clinical trials on bronchiectasis have prioritized exacerbation as the primary outcome (12,51,55). The endotypes of bronchiectasis exacerbation, which may provide insights into the underlying biological mechanisms and potential therapeutic solutions, are discussed later in this review.
High symptom burden phenotype
Current bronchiectasis guidelines regard the prevention of exacerbation and the management of daily symptoms as separate objectives. However, Gao et al. questioned this notion, noting that exacerbations worsen daily symptoms. Therefore, worse daily symptoms are a risk factor for exacerbation (56). The study revealed that the exacerbation risk increases by 10% for each 10-unit increase in the score on St. George’s Respiratory Questionnaire, a validated health-related quality of life questionnaire (56). Furthermore, this observation was replicated in a prospective cohort study (n=436), which evaluated the symptom burden using the Quality-of-Life Bronchiectasis Respiratory Symptom Scale (QOL-B-RSS) (57). The baseline QOL-B-RSS score was associated with an increased risk of exacerbations (rate ratio: 1.3 for each 10-point decrease), hospitalizations (rate ratio: 1.2), and reduced time to the first exacerbation (hazard ratio: 1.1) over 12 months, even after adjusting for potential confounders such as exacerbation history (58).
As highly symptomatic patients with bronchiectasis have an increased risk of exacerbation, hospitalization, and poor clinical outcomes, the high symptom burden phenotype should be regarded as a distinct phenotype. Accumulating more evidence related to the management of highly symptomatic patients with bronchiectasis could lead to a shift in the paradigm of bronchiectasis management, potentially influencing future bronchiectasis guidelines (58).
Martinez-Garcia et al. previously proposed a clinical fingerprint for bronchiectasis, which comprised three components; activity (e.g., bronchial bacterial infection and number of annual exacerbations), impact (e.g., health-related quality of life and degree of dyspnea), and severity [e.g., forced expiratory volume in one second (FEV1) and radiological extent]. This fingerprint may help physicians identify the clinical impact of the disease more precisely while taking into account the symptom burden (59).
Chronic airway diseases overlapped phenotype
International bronchiectasis registries consistently reported that COPD and asthma are common in patients with bronchiectasis, ranging from 29–60%, although the registries enrolled patients whose primary diagnosis was bronchiectasis (19-24). The COPD-bronchiectasis overlap is clinically relevant because patients with this overlap are more likely to experience more severe symptoms, poorer quality of life, more frequent exacerbations, and increased mortality than those with COPD or bronchiectasis alone (60-64). Similarly, the asthma-bronchiectasis overlap has been associated with an increased risk of exacerbations (65), improved outcomes from ICS use, and reduced mortality, as indicated in a recent European Multicenter Bronchiectasis Audit and Research Collaboration (EMBARC) report (66).
However, there has been confusion over the diagnosis of COPD-bronchiectasis overlap because bronchiectasis is frequently observed in patients with COPD, and bronchiectasis can also cause airflow limitation (25,67,68). To address this diagnostic challenge, an international consensus proposed the standardized ROSE criteria {radiological bronchiectasis (R), obstruction [FEV1/FVC (forced vital capacity) ratio <0.7; O], symptoms (S), and exposure ≥10 pack-years of smoking (E)} (69). Significantly, a recent EMBARC study (n=16,730) revealed that 4,366 had a clinician-assigned COPD diagnosis; however, 22% of them did not have airflow obstruction, and 32% did not have a history of ≥10 pack-years of smoking (25). Hence, the ROSE criteria (25,69), a multi-omics study (discussed later) (18), and future studies would elucidate a more accurate diagnosis and reveal treatable traits in patients with COPD-bronchiectasis overlap.
Asthma-bronchiectasis overlap presents additional complexities in clinical practice. Diagnosing this overlap would be more challenging than the COPD-bronchiectasis overlap. Tests, such as bronchodilator reversibility, peak expiratory flow rate variability, fractional exhaled nitric oxide (FeNO) levels, and bronchial hyperresponsiveness, lack the sensitivity and specificity required for a definitive asthma diagnosis, although airflow obstruction to define COPD is relatively straightforward (70,71). Therefore, the misdiagnosis of asthma is prevalent in the general population, including those with bronchiectasis.
Disease labels are constructed to categorize patients for treatment. However, this classification system can be problematic for complex airway diseases. Approximately 50% of the patients with a single condition, such as COPD or asthma, may have bronchiectasis. Eventually, care for patients with severe and complex airway diseases may shift towards an endotype-driven approach (72). This strategy focuses on treating the underlying inflammatory pathways, such as Th2 airway inflammation, with medications, such as ICS or Th2 inflammation-targeting biologics, regardless of the primary diagnosis. Additionally, future treatments may specifically target neutrophilic inflammation, such as DPP-1 inhibitors, which are currently under clinical development for bronchiectasis (2).
Phenotypes identified by clustering studies
Unsupervised cluster analyses based on clinical characteristics were also performed to determine the different bronchiectasis phenotypes. A European multicenter registry study (n=1,145) identified four clusters: severe disease with P. aeruginosa infection, chronic infection with other pathogens, sputum production without chronic bacterial infection, and dry bronchiectasis (73). Similarly, a Chinese study (n=148) identified four clusters: younger patients with mild and idiopathic bronchiectasis; older patients with severe post-infective bronchiectasis and P. aeruginosa infection; older patients with late-onset, severe idiopathic bronchiectasis; and older patients with moderate disease (74). Additionally, a Spanish study (n=468) revealed four clusters: young women with mild disease; overweight elderly women with mild bronchiectasis; elderly men with severe disease, chronic P. aeruginosa infection, airflow obstruction, and exacerbations; and older patients with severe disease and infrequent exacerbations (75).
Notably, patients with bronchiectasis and P. aeruginosa infection consistently exhibited distinct characteristics in all three studies (73-75), discussed earlier. However, other phenotypes identified by cluster analyses, including age of onset, idiopathic etiology, and overweight status, were too non-specific and had limited value in advancing the understanding of definitive bronchiectasis phenotypes.
Endotypes in bronchiectasis
Neutrophilic inflammation
Neutrophilic inflammation is a primary driver of bronchiectasis severity and progression, with high airway neutrophil levels associated with various chemokines, such as leukotriene B4, interleukin (IL)-8, IL-1β, and tumor necrosis factor-α (76). These neutrophils exhibit functional abnormalities, including excessive protease release, overwhelming anti-protease defense, and impaired pathogen clearance (77,78). Neutrophil extracellular trap (NET) formation, which eliminates pathogens, can lead to tissue damage and inflammation. NETs release neutrophil elastase (NE), a key enzyme involved in tissue degranulation, impaired bacterial clearance, and mucus hypersecretion. Sputum NE levels correlate with exacerbation risk, lung function decline, and mortality (11,79). The NEATstik test is used to measure sputum NE levels and identify patients at a higher risk of exacerbation (80). Notably, a recent European multicenter study (n=13,484) demonstrated that sputum color, a more accessible measure of neutrophilic inflammation, was strongly associated with disease severity, lower FEV1, worse quality of life, and greater radiological severity of bronchiectasis (81).
DPP-1, crucial for activating serine proteases such as NE, is inhibited by drugs like brensocatib (78). The WILLOW trial demonstrated that brensocatib prolonged the time to the first exacerbation and reduced NE activity (12). Additionally, the ongoing phase 3 ASPEN trial showed a 20% reduction in the frequency of exacerbations (ClinicalTrials.gov: NCT04594369) (50), further confirming its efficacy. Another DPP-1 inhibitor, BI 1291583, demonstrated a numerical reduction in exacerbation risk (82) in an ongoing phase 2 trial (NCT03696290). Identifying patients with bronchiectasis and increased neutrophilic inflammation based on NET or NE levels could enable targeted therapies. Further studies are needed to refine the markers of neutrophilic inflammatory endotypes and guide precision treatments.
Eosinophilic inflammation
Although bronchiectasis has traditionally been regarded as a neutrophilic disease, it involves eosinophilic inflammation in some patients. One study reported blood eosinophilia in 22.6% of 1,007 patients, correlating with sputum eosinophilia and Th2 inflammation (83). High eosinophil counts are associated with increased disease severity and mortality, with eosinophilic patients experiencing shorter exacerbation-free periods after antibiotic treatment (83).
FeNO serves as a Th2 inflammation marker, with a Th2-high endotype noted in 31% of non-asthmatic bronchiectasis patients (84). This endotype is associated with higher disease severity and lower lung function. Identifying the eosinophilic endotype through blood and FeNO tests underscores the potential of precision medicine.
Monoclonal antibodies targeting the Th2 pathway, which have been proven effective in treating eosinophilic asthma, are yet to be tested in patients with bronchiectasis. Initial trials with inhaled fluticasone suggested improvements in the quality of life of patients with eosinophilic bronchiectasis (85). A case series indicated the benefits of mepolizumab and benralizumab in patients with high eosinophil counts (10). Preliminary trials, including an initial benralizumab trial (NCT05006573), are underway to provide promising insights into the efficacy of these biologics in the treatment of bronchiectasis.
Systemic inflammation
In bronchiectasis, inflammation is primarily localized in the lungs; however, systemic inflammation is observed during stable phases and exacerbations, correlating with disease severity. Clinical studies have shown that white blood cell and neutrophil counts increase during exacerbations, decrease with treatment, and align with radiological severity and lung function decline (86,87). A total of 802 patients from the Spanish Bronchiectasis Registry showed persistently high C-reactive protein (CRP) levels during severe exacerbations, predicting bacterial infections and poor outcomes following antibiotic treatment (88).
Fibrinogen, a coagulation cascade component, is a biomarker for the proinflammatory phenotype in COPD (89). In bronchiectasis, fibrinogen levels are also correlated with higher Bronchiectasis Severity Index; FACED (FEV1, age, chronic colonization, radiological extension, and dyspnea) score, and exacerbation risk (90). Saleh et al. analyzed 31 proteins in 90 patients and identified fibrinogen as a key severity marker associated with poor lung function and P. aeruginosa colonization (91). Additionally, the erythrocyte sedimentation rate, largely influenced by fibrinogen concentration, is correlated with exacerbation frequency and treatment response (86).
Desmosine, a product of elastin degradation by NE, is correlated with sputum elastase levels and severe exacerbations of bronchiectasis (79). It is linked to atherosclerosis and poorer post-myocardial infarction outcomes. Huang et al. further found that elevated serum desmosine levels were linked to increased cardiovascular mortality in these patients (92).
Systemic inflammation promotes platelet aggregation. In a study by Aliberti et al. (93), the occurrence of thrombocytosis in 1,771 patients with bronchiectasis was related to severe disease, poorer quality of life, increased frequency of exacerbations, and higher mortality at 3 and 5 years. Méndez et al. (94) reported elevated soluble P-selectin levels in patients with severe cases, indicating heightened platelet activation at baseline.
Cluster analyses that incorporate multiple systemic biomarkers may provide a more effective approach to characterizing patients with bronchiectasis compared with the measurement of individual biomarkers alone. Wang et al. (95) identified three distinct clusters according to the levels of systemic biomarkers, including neutrophils, eosinophils, lymphocyte counts, CRP, and hemoglobin, which exhibited significant correlations with disease severity. However, several clinical factors, such as comorbidities, affect systemic inflammatory markers. Therefore, we should understand that systemic inflammation exists in bronchiectasis, but it is challenging to recognize its clinical significance precisely and treat it as a treatable target, unlike airway inflammation.
Antimicrobial peptides (AMPs)
AMPs are a diverse group of molecules that play a crucial role in host defense against microbial infections; however, they can exhibit pro-inflammatory properties in the context of chronic lung disease. Sibila et al. (96) found that frequent exacerbators with bronchiectasis exhibited dysregulated levels of sputum AMPs, characterized by elevated levels of LL-37 and decreased levels of secretory leukocyte peptidase inhibitors (SLPIs). Elevated LL-37 and diminished SLPI levels in the sputum were independently associated with disease severity, P. aeruginosa infection, and an increased risk of future exacerbations. Furthermore, cluster analysis conducted by Perea et al. demonstrated that patients could be classified according to their AMP levels, with the highest concentrations associated with the most severe disease presentations (97).
Endotypes identified by clustering studies
Given the heterogeneity of the causes, clinical manifestations, and treatment responses in patients with bronchiectasis, multiple inflammatory endotypes have been identified and classified according to their distinct biological mechanisms. Choi et al. (98) recently delineated four inflammatory endotypes using unsupervised cluster analysis based on 20 sputum and 13 serum inflammatory markers: (I) milder neutrophilic inflammation, characterized by lower concentrations of inflammatory markers, reduced exacerbation rates, and less pronounced airway microbiome dysbiosis; (II) mixed neutrophilic and Th2 inflammation, defined by the presence of biomarkers such as IL-5, IL-8, and NE; (III) severe neutrophilic inflammation, characterized by elevated levels of IL-8, NE, and NETs, with both clusters 2 and 3 associated with increased exacerbation risk and Pseudomonas-enriched microbiome; and (IV) mixed epithelial and Th2 inflammation, characterized by presence of biomarkers such as IL-5, monocyte chemoattractant protein-1, IL-6, and vascular endothelial growth factor. This endotype was associated with a Streptococcus-dominated microbiome and systemic inflammation. The associations between these specific inflammatory endotypes, infections, and clinical outcomes were further corroborated by previous evidence linking NE and microbial infections to poor outcomes. Additionally, severe disease has been observed in patients with eosinophilia, albeit with distinct microbiome profiles.
Regarding COPD-bronchiectasis overlap, Huang et al. (18) found that patients with this overlap exhibited distinct lung microbiota and host response profiles compared with patients with COPD alone, whose microbiota resembled that of patients with bronchiectasis. The authors proposed five endotypes based on clinical characteristics, sputum microbiome profiles, and protein markers that reflected potential “treatable traits”. These endotypes include (I) diverse-protective endotype: associated with the most favorable prognosis; (II) Haemophilus-proteolytic endotype: linked to Haemophilus infection, with tetracyclines as a potential treatment option; (III) infected-epithelial response endotype: characteristic of patients with bronchiectasis with gram-negative infections, likely benefiting from macrolide therapy; (IV) Proteobacteria-neutrophilic endotype: possibly responsive to macrolides due to similarities with bronchiectasis, including heightened neutrophil activation and NET formation; and (V) Th2 endotype: noted for its responsiveness to ICS or other therapies targeting Th2-mediated inflammation.
Taken together, precise endotype analysis that accounts for distinct causes and inflammatory profiles enhances clinical decision-making in the management of bronchiectasis.
Microbiome
Airway infections play a fundamental role in the pathogenesis of bronchiectasis. Bacterial infections have traditionally been attributed to pathogenic organisms. However, the introduction of next-generation sequencing has shifted this perspective, highlighting microbial dysbiosis, characterized by a loss of microbial diversity and the dominance of certain organisms, as a significant factor in disease progression (99).
Recent studies have revealed that bronchiectasis is often associated with a Proteobacteria-dominant microbiome, particularly featuring Pseudomonas, which is linked to more severe diseases and poorer clinical outcomes (98,100). This suggests that targeting specific microbial populations may offer potential avenues for intervention (100). Some patients exhibit a Firmicutes-dominant microbiome, such as Streptococci. The decline in commensal anti-inflammatory bacteria such as Rothia mucilaginosa has important clinical implications (101). Microbiomes also harbor resistome and antimicrobial resistance genes, which can guide the development of targeted therapeutic strategies (102). Additionally, the mycobiome, particularly of Aspergillus, is clinically relevant owing to its role in fungal sensitization and immune responses (103).
A key question remains whether reduced microbial diversity leads to increased risk of exacerbations and worsened outcomes due to the overgrowth of dominant pathogens or whether it is a result of repeated antibiotic treatments causing microbial depletion and subsequent colonization. Notably, approximately one-third of patients with bronchiectasis do not exhibit chronic infection; instead, they have a microbiome enriched with “commensals” such as Streptococcus, Veillonella, Prevotella, Rothia, and Neisseria (99,101,104). Understanding how these taxa may act as “pathobionts” under certain conditions is vital.
Future research should focus on the dynamics of microbial communities rather than evaluating isolated microbes. Investigating how the microbiome interacts with clinical, inflammatory, immunological, and metabolic factors may open new avenues for understanding and treating bronchiectasis.
Genetics
Genetic investigations specific to bronchiectasis are limited. Whole-genome sequencing (WGS) is expected to uncover novel genetic etiologies in patients with idiopathic bronchiectasis. The Genetics Encoding Complex Ciliopathies of Bronchiectasis study, conducted by EMBARC, analyzed whole exome sequences from over 1,000 patients with idiopathic bronchiectasis. Preliminary data indicated that WGS detected PCD in 12% of patients with severe bronchiectasis and 7% of those not typically suspected of this condition (17).
Mannose-binding lectin, an essential component of the innate immune response, is commonly genetically deficient, and this deficiency is associated with increased disease severity, poor quality of life, higher exacerbation rates, and more frequent hospitalizations (105). Additionally, the secretion of α-1,2-fucosylated glycans, regulated by the secretor genotype, has emerged as a risk factor influencing infection type and disease progression (106).
As larger patient cohorts with linked biobanks are established, a more comprehensive understanding of the genetic factors that contribute to bronchiectasis endotypes is essential. Moreover, investigating the ethnic and geographical variations in genetic variants will provide valuable insights into their roles in the pathogenesis of bronchiectasis.
Endotypes of bronchiectasis exacerbation
Bronchiectasis exacerbations have traditionally been regarded as bacterial infection-driven events; therefore, they are primarily managed with antibiotics (13,15). However, this simplistic view has been challenged by the heterogeneity of exacerbations, which can arise from various exogenous and endogenous factors that disrupt patient homeostasis.
Gao et al. (107) proposed that bronchiectasis exacerbations can be categorized into distinct types: bacterial, viral, bacterial/viral co-infection, eosinophilic, and unidentified types, each with unique microbiome and inflammatory profiles. They identified four exacerbation endotypes through an integrative approach that combined microbiome analysis, proteomics, and viral polymerase chain reaction: (I) a Pseudomonas-associated cluster, characterized by significant upregulation of neutrophil proteins such as ELANE (elastase), myeloperoxidase, and azurocidin; (II) a Haemophilus-associated cluster, marked by elevated levels of human defensins (defensin alpha 1/3); (III) an eosinophilic inflammation cluster associated with proteins like EZR, PYCARD, WHAG, and Galectin-10, indicating non-infective exacerbations; and (IV) a cluster defined by excessive mucus production and epithelial response, with high expression of MUC5AC, MUC5B, and PIGR, and a Streptococcus-dominated microbiome.
Understanding these exacerbation endotypes is critical for selecting suitable patients for clinical trials and developing precise treatment strategies. Ongoing research on biomarkers defining these endotypes is expected to enhance personalized prevention and therapy for bronchiectasis exacerbation.
Conclusions
Toward precision medicine
The heterogeneity of bronchiectasis poses significant challenges in disease management, complicating efforts to tailor treatments and improve patient outcomes. Although the concept of treatable traits in bronchiectasis provides a strategic approach to address this challenge, it often lacks critical biological insights into the underlying causal mechanisms that are essential for developing novel, targeted interventions. Insights gained from other lung conditions, such as asthma and COPD, highlight that integrating endophenotyping can significantly enhance the understanding of disease characteristics, thereby enabling the development of more precise and effective therapeutic strategies. Figure 2 illustrates how determining phenotypes and endotypes contributes to developing individualized treatment strategies for bronchiectasis.
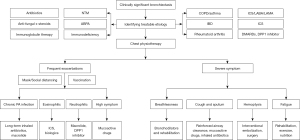
In bronchiectasis, clinical phenotypes involve categorizing the disease based on patient characteristics; however, this process remains complex. Widely recognized clinical phenotypes, such as “NTM-PD”, “chronic P. aeruginosa infection”, “frequent exacerbator”, “high symptom burden”, and “chronic airway disease overlapped”, provide clear clinical relevance but only capture part of the disease’s complexity. Further phenotypic layers involve the analysis of radiographic patterns and the identification of underlying etiologies. However, clinical phenotyping alone is inadequate to fully encapsulate the complexity of the disease, which involves differences in severity, activity, and impact among different phenotypes.
Investigating molecular endotypes that evaluate the disease based on the underlying pathobiological mechanisms and treatment responses provides a more comprehensive understanding of bronchiectasis. This approach is exemplified by the identification of distinct inflammatory patterns, such as neutrophilic/NETS and eosinophilic/Th2 airway inflammation. Each clinical phenotype may encompass multiple endotypes, and a single endotype may be associated with multiple phenotypes. Identifying the therapeutic targets within these frameworks introduces the concept of “treatable traits”. Advancing endophenotyping in bronchiectasis, is therefore, vital for applying precision medicine strategies that more effectively tackle the considerable heterogeneity of the disease, ultimately enhancing patient outcomes.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Prof. James D Chalmers and Prof. Wei-Jie Guan) for the series “Frontiers in Bronchiectasis Management: Translational Science and Practice” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1945/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1945/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1945/coif). The series “Frontiers in Bronchiectasis Management: Translational Science and Practice” was commissioned by the editorial office without any funding or sponsorship. H.C. reports grant from the Basic Science Research Program of the Korean Ministry of Education (grant No. 2021R1I1A3052416); lecture fees from Boryung Pharmaceutical Co., Kolon Pharma and Abbott. Y.H.G. reports grants from the National Natural Science Foundation of China (grant No. 82270047) and Noncommunicable Chronic Diseases-National Science and Technology Major Project (grant No. 2024ZD0529700). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chalmers JD, Goeminne PC, Ringshausen FC. EMBARCing on a new era for bronchiectasis: a review series for the Seventh World Bronchiectasis Conference. Eur Respir Rev 2024;33:240124. [Crossref] [PubMed]
- Choi H, McShane PJ, Aliberti S, et al. Bronchiectasis management in adults: state of the art and future directions. Eur Respir J 2024;63:2400518. [Crossref] [PubMed]
- Choi H, Chalmers JD. Bronchiectasis: from orphan disease to precision medicine. In: Wagner TOF, Humbert M, Wijsenbeek M, et al., eds. Rare Diseases of the Respiratory System (ERS Monograph). Sheffield: European Respiratory Society; 2023:150-64.
- Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers 2018;4:45. [Crossref] [PubMed]
- O'Donnell AE. Bronchiectasis - A Clinical Review. N Engl J Med 2022;387:533-45. [Crossref] [PubMed]
- Chalmers JD. Phenotypes and endotypes. In: Chalmers JD, Polverino E, Aliberti S, editors. Bronchiectasis (ERS Monograph). Sheffield: European Respiratory Society 2018:133-52.
- Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182:598-604. [Crossref] [PubMed]
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372:1107-19. [Crossref] [PubMed]
- Martínez-García MÁ, Méndez R, Olveira C, et al. The U-Shaped Relationship Between Eosinophil Count and Bronchiectasis Severity: The Effect of Inhaled Corticosteroids. Chest 2023;164:606-13. [Crossref] [PubMed]
- Rademacher J, Konwert S, Fuge J, et al. Anti-IL5 and anti-IL5Rα therapy for clinically significant bronchiectasis with eosinophilic endotype: a case series. Eur Respir J 2020;55:1901333. [Crossref] [PubMed]
- Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021;9:873-84. [Crossref] [PubMed]
- Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N Engl J Med 2020;383:2127-37. [Crossref] [PubMed]
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629. [Crossref] [PubMed]
- Agarwal R, Sehgal IS, Muthu V, et al. Revised ISHAM-ABPA working group clinical practice guidelines for diagnosing, classifying and treating allergic bronchopulmonary aspergillosis/mycoses. Eur Respir J 2024;63:2400061. [Crossref] [PubMed]
- Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019;74:1-69. [Crossref] [PubMed]
- De Soyza A, McDonnell MJ, Goeminne PC, et al. Bronchiectasis Rheumatoid Overlap Syndrome Is an Independent Risk Factor for Mortality in Patients With Bronchiectasis: A Multicenter Cohort Study. Chest 2017;151:1247-54. [Crossref] [PubMed]
- Shoemark A, Griffin H, Wheway G, et al. Genome sequencing reveals underdiagnosis of primary ciliary dyskinesia in bronchiectasis. Eur Respir J 2022;60:2200176. [Crossref] [PubMed]
- Huang JT, Cant E, Keir HR, et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the "Chronic Obstructive Pulmonary Disease-Bronchiectasis Association". Am J Respir Crit Care Med 2022;206:417-26. [Crossref] [PubMed]
- Aksamit TR, O'Donnell AE, Barker A, et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017;151:982-92. [Crossref] [PubMed]
- Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med 2023;11:637-49. [Crossref] [PubMed]
- Dhar R, Singh S, Talwar D, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019;7:e1269-79. [Crossref] [PubMed]
- Lee H, Choi H, Chalmers JD, et al. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021;26:619-21. [Crossref] [PubMed]
- Visser SK, Bye PTP, Fox GJ, et al. Australian adults with bronchiectasis: The first report from the Australian Bronchiectasis Registry. Respir Med 2019;155:97-103. [Crossref] [PubMed]
- Choi H, Xu JF, Chotirmall SH, et al. Bronchiectasis in Asia: a review of current status and challenges. Eur Respir Rev 2024;33:240096. [Crossref] [PubMed]
- Polverino E, De Soyza A, Dimakou K, et al. The Association between Bronchiectasis and Chronic Obstructive Pulmonary Disease: Data from the European Bronchiectasis Registry (EMBARC). Am J Respir Crit Care Med 2024;210:119-27. [Crossref] [PubMed]
- Kunst H, Wickremasinghe M, Wells A, et al. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur Respir J 2006;28:352-7. [Crossref] [PubMed]
- Máiz L, Girón R, Olveira C, et al. Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: a multicenter observational study. BMC Infect Dis 2016;16:437. [Crossref] [PubMed]
- Shteinberg M, Stein N, Adir Y, et al. Prevalence, risk factors and prognosis of nontuberculous mycobacterial infection among people with bronchiectasis: a population survey. Eur Respir J 2018;51:1702469. [Crossref] [PubMed]
- Suska K, Amati F, Sotgiu G, et al. Nontuberculous mycobacteria infection and pulmonary disease in bronchiectasis. ERJ Open Res 2022;8:00060-2022. [Crossref] [PubMed]
- Yang B, Ryu J, Kim T, et al. Impact of Bronchiectasis on Incident Nontuberculous Mycobacterial Pulmonary Disease: A 10-Year National Cohort Study. Chest 2021;159:1807-11. [Crossref] [PubMed]
- Choi H, Han K, Yang B, et al. Female Reproductive Factors and Incidence of Nontuberculous Mycobacterial Pulmonary Disease Among Postmenopausal Women in Korea. Clin Infect Dis 2022;75:1397-404. [Crossref] [PubMed]
- Lee H, Choi H. Female Hormone Exposure and Gastroesophageal Reflux Disease Are Also Potential Risk Factors for Nontuberculous Mycobacterial Pulmonary Disease. Chest 2023;164:e155-6. [Crossref] [PubMed]
- Loebinger MR, Quint JK, van der Laan R, et al. Risk Factors for Nontuberculous Mycobacterial Pulmonary Disease: A Systematic Literature Review and Meta-Analysis. Chest 2023;164:1115-24. [Crossref] [PubMed]
- Dettmer S, Ringshausen FC, Fuge J, et al. Computed Tomography in Adults with Bronchiectasis and Nontuberculous Mycobacterial Pulmonary Disease: Typical Imaging Findings. J Clin Med 2021;10:2736. [Crossref] [PubMed]
- Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 2016;45:123-34. [Crossref] [PubMed]
- Choi H, Hughes C, Eke Z, et al. Clinical Efficacy of Serum Antiglycopeptidolipid Core IgA Antibody Test for Screening Nontuberculous Mycobacterial Pulmonary Disease in Bronchiectasis: A European Multicenter Cohort Study. Chest 2024;S0012-3692(24)05418-7.
- Aksamit TR, Locantore N, Addrizzo-Harris D, et al. Five-Year Outcomes among U.S. Bronchiectasis and NTM Research Registry Patients. Am J Respir Crit Care Med 2024;210:108-18. [Crossref] [PubMed]
- Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018;51:1701953. [Crossref] [PubMed]
- Finch S, McDonnell MJ, Abo-Leyah H, et al. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann Am Thorac Soc 2015;12:1602-11. [Crossref] [PubMed]
- Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009;34:843-9. [Crossref] [PubMed]
- Davies G, Wells AU, Doffman S, et al. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J 2006;28:974-9. [Crossref] [PubMed]
- McDonnell MJ, Jary HR, Perry A, et al. Non cystic fibrosis bronchiectasis: A longitudinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respir Med 2015;109:716-26. [Crossref] [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [Crossref] [PubMed]
- Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357-67. [Crossref] [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Effect of airway Pseudomonas aeruginosa isolation and infection on steady-state bronchiectasis in Guangzhou, China. J Thorac Dis 2015;7:625-36. [Crossref] [PubMed]
- Song J, Sin S, Kang HR, et al. Clinical Impacts of Pseudomonas aeruginosa Isolation in Patients with Bronchiectasis: Findings from KMBARC Registry. J Clin Med 2024;13:5011. [Crossref] [PubMed]
- Cordeiro R, Choi H, Haworth CS, et al. The Efficacy and Safety of Inhaled Antibiotics for the Treatment of Bronchiectasis in Adults: Updated Systematic Review and Meta-Analysis. Chest 2024;166:61-80. [Crossref] [PubMed]
- Conceição M, Shteinberg M, Goeminne P, et al. Eradication treatment for Pseudomonas aeruginosa infection in adults with bronchiectasis: a systematic review and meta-analysis. Eur Respir Rev 2024;33:230178. [Crossref] [PubMed]
- Gao YH, Lu HW, Zheng HZ, et al. A phase 4 multicentre, 2×2 factorial randomised, double-blind, placebo-controlled trial to investigate the efficacy and safety of tobramycin inhalation solution for Pseudomonas aeruginosa eradication in bronchiectasis: ERASE. ERJ Open Res 2024; [Crossref]
- Chalmers JD, Burgel PR, Daley CL, et al. Brensocatib in non-cystic fibrosis bronchiectasis: ASPEN protocol and baseline characteristics. ERJ Open Res 2024;10:00151-2024. [Crossref] [PubMed]
- Chalmers JD, Gupta A, Chotirmall SH, et al. A Phase 2 randomised study to establish efficacy, safety and dosing of a novel oral cathepsin C inhibitor, BI 1291583, in adults with bronchiectasis: Airleaf. ERJ Open Res 2023;9:00633-2022. [Crossref] [PubMed]
- Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the "Frequent Exacerbator Phenotype" in Bronchiectasis. Am J Respir Crit Care Med 2018;197:1410-20. [Crossref] [PubMed]
- Choi H, Chalmers JD. Bronchiectasis exacerbation: a narrative review of causes, risk factors, management and prevention. Ann Transl Med 2023;11:25. [Crossref] [PubMed]
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [Crossref] [PubMed]
- Haworth CS, Shteinberg M, Winthrop K, et al. Inhaled colistimethate sodium in patients with bronchiectasis and Pseudomonas aeruginosa infection: results of PROMIS-I and PROMIS-II, two randomised, double-blind, placebo-controlled phase 3 trials assessing safety and efficacy over 12 months. Lancet Respir Med 2024;12:787-98. [Crossref] [PubMed]
- Gao YH, Abo Leyah H, Finch S, et al. Relationship between Symptoms, Exacerbations, and Treatment Response in Bronchiectasis. Am J Respir Crit Care Med 2020;201:1499-507. [Crossref] [PubMed]
- Gao YH, Zheng HZ, Lu HW, et al. Quality-of-Life Bronchiectasis Respiratory Symptom Scale Predicts the Risk of Exacerbations in Adults with Bronchiectasis: A Prospective Observational Study. Ann Am Thorac Soc 2024;21:393-401. [Crossref] [PubMed]
- Im Y, Chalmers JD, Choi H. Disease Severity and Activity in Bronchiectasis: A Paradigm Shift in Bronchiectasis Management. Tuberc Respir Dis (Seoul) 2025;88:109-19. [Crossref] [PubMed]
- Martinez-Garcia MA, Aksamit TR, Agusti A. Clinical Fingerprinting: A Way to Address the Complexity and Heterogeneity of Bronchiectasis in Practice. Am J Respir Crit Care Med 2020;201:14-9. [Crossref] [PubMed]
- Choi H, Lee H, Ryu J, et al. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: a nationwide population study. Ther Adv Respir Dis 2020;14:1753466620963030. [Crossref] [PubMed]
- Choi H, Yang B, Kim YJ, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep 2021;11:7126. [Crossref] [PubMed]
- Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J 2018;52:1800328. [Crossref] [PubMed]
- Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2015;10:1465-75. [Crossref] [PubMed]
- McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016;4:969-79. [Crossref] [PubMed]
- Mao B, Yang JW, Lu HW, et al. Asthma and bronchiectasis exacerbation. Eur Respir J 2016;47:1680-6. [Crossref] [PubMed]
- Polverino E, Dimakou K, Traversi L, et al. Bronchiectasis and asthma: Data from the European Bronchiectasis Registry (EMBARC). J Allergy Clin Immunol 2024;153:1553-62. [Crossref] [PubMed]
- Everaerts S, McDonough JE, Verleden SE, et al. Airway morphometry in COPD with bronchiectasis: a view on all airway generations. Eur Respir J 2019;54:1802166. [Crossref] [PubMed]
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:400-7. [Crossref] [PubMed]
- Traversi L, Miravitlles M, Martinez-Garcia MA, et al. ROSE: radiology, obstruction, symptoms and exposure - a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res 2021;7:00399-2021. [Crossref] [PubMed]
- Tiotiu A, Martinez-Garcia MA, Mendez-Brea P, et al. Does asthma-bronchiectasis overlap syndrome (ABOS) really exist? J Asthma 2023;60:1935-41. [Crossref] [PubMed]
- Kim SH, Yang B, Min KH, et al. Defining the overlap between asthma and bronchiectasis: A call for consensus definition. J Allergy Clin Immunol 2024;154:1560-1. [Crossref] [PubMed]
- Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016;47:410-9. [Crossref] [PubMed]
- Aliberti S, Lonni S, Dore S, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016;47:1113-22. [Crossref] [PubMed]
- Guan WJ, Jiang M, Gao YH, et al. Unsupervised learning technique identifies bronchiectasis phenotypes with distinct clinical characteristics. Int J Tuberc Lung Dis 2016;20:402-10. [Crossref] [PubMed]
- Martínez-García MÁ, Vendrell M, Girón R, et al. The Multiple Faces of Non-Cystic Fibrosis Bronchiectasis. A Cluster Analysis Approach. Ann Am Thorac Soc 2016;13:1468-75. [Crossref] [PubMed]
- Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol 2013;55:27-34. [Crossref] [PubMed]
- Giam YH, Shoemark A, Chalmers JD. Neutrophil dysfunction in bronchiectasis: an emerging role for immunometabolism. Eur Respir J 2021;58:2003157. [Crossref] [PubMed]
- Chalmers JD, Mall MA, Chotirmall SH, et al. Targeting neutrophil serine proteases in bronchiectasis. Eur Respir J 2025;65:2401050. [Crossref] [PubMed]
- Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am J Respir Crit Care Med 2017;195:1384-93. [Crossref] [PubMed]
- Shoemark A, Cant E, Carreto L, et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur Respir J 2019;53:1900303. [Crossref] [PubMed]
- Aliberti S, Ringshausen FC, Dhar R, et al. Objective sputum colour assessment and clinical outcomes in bronchiectasis: data from the European Bronchiectasis Registry (EMBARC). Eur Respir J 2024;63:2301554. [Crossref] [PubMed]
- Chalmers JD, Shteinberg M, Mall MA, et al. Cathepsin C (dipeptidyl peptidase 1) inhibition in adults with bronchiectasis: AIRLEAF, a phase II randomised, double-blind, placebo-controlled, dose-finding study. Eur Respir J 2025;65:2401551. [Crossref] [PubMed]
- Shoemark A, Shteinberg M, De Soyza A, et al. Characterization of Eosinophilic Bronchiectasis: A European Multicohort Study. Am J Respir Crit Care Med 2022;205:894-902. [Crossref] [PubMed]
- Oriano M, Gramegna A, Amati F, et al. T2-High Endotype and Response to Biological Treatments in Patients with Bronchiectasis. Biomedicines 2021;9:772. [Crossref] [PubMed]
- Aliberti S, Sotgiu G, Blasi F, et al. Blood eosinophils predict inhaled fluticasone response in bronchiectasis. Eur Respir J 2020;56:2000453. [Crossref] [PubMed]
- Wilson CB, Jones PW, O'Leary CJ, et al. Systemic markers of inflammation in stable bronchiectasis. Eur Respir J 1998;12:820-4. [Crossref] [PubMed]
- Coban H, Gungen AC. Is There a Correlation between New Scoring Systems and Systemic Inflammation in Stable Bronchiectasis? Can Respir J 2017;2017:9874068. [Crossref] [PubMed]
- Posadas T, Oscullo G, Zaldivar E, et al. C-Reactive Protein Concentration in Steady-State Bronchiectasis: Prognostic Value of Future Severe Exacerbations. Data From the Spanish Registry of Bronchiectasis (RIBRON). Arch Bronconeumol (Engl Ed) 2021;57:21-7. [Crossref] [PubMed]
- Miller BE, Tal-Singer R, Rennard SI, et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am J Respir Crit Care Med 2016;193:607-13. [Crossref] [PubMed]
- Lee SJ, Jeong JH, Heo M, et al. Serum Fibrinogen as a Biomarker for Disease Severity and Exacerbation in Patients with Non-Cystic Fibrosis Bronchiectasis. J Clin Med 2022;11:3948. [Crossref] [PubMed]
- Saleh AD, Chalmers JD, De Soyza A, et al. The heterogeneity of systemic inflammation in bronchiectasis. Respir Med 2017;127:33-9. [Crossref] [PubMed]
- Huang JT, Kuzmanova E, Dicker AJ, et al. Serum Desmosine Is Associated with Long-Term All-Cause and Cardiovascular Mortality in Bronchiectasis. Am J Respir Crit Care Med 2020;202:897-9. [Crossref] [PubMed]
- Aliberti S, Sotgiu G, Gramegna A, et al. Thrombocytosis during Stable State Predicts Mortality in Bronchiectasis. Ann Am Thorac Soc 2021;18:1316-25. [Crossref] [PubMed]
- Méndez R, Moscardó A, Latorre A, et al. Soluble P-Selectin in Acute Exacerbations and Stable Bronchiectasis in Adults. Ann Am Thorac Soc 2019;16:1587-91. [Crossref] [PubMed]
- Wang X, Villa C, Dobarganes Y, et al. Systemic Inflammatory Biomarkers Define Specific Clusters in Patients with Bronchiectasis: A Large-Cohort Study. Biomedicines 2022;10:225. [Crossref] [PubMed]
- Sibila O, Perea L, Cantó E, et al. Antimicrobial peptides, disease severity and exacerbations in bronchiectasis. Thorax 2019;74:835-42. [Crossref] [PubMed]
- Perea L, Cantó E, Suarez-Cuartin G, et al. A Cluster Analysis of Bronchiectasis Patients Based on the Airway Immune Profile. Chest 2021;159:1758-67. [Crossref] [PubMed]
- Choi H, Ryu S, Keir HR, et al. Inflammatory Molecular Endotypes in Bronchiectasis: A European Multicenter Cohort Study. Am J Respir Crit Care Med 2023;208:1166-76. [Crossref] [PubMed]
- Richardson H, Dicker AJ, Barclay H, et al. The microbiome in bronchiectasis. Eur Respir Rev 2019;28:190048. [Crossref] [PubMed]
- Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med 2021;9:885-96. [Crossref] [PubMed]
- Rigauts C, Aizawa J, Taylor SL, et al. R othia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur Respir J 2022;59:2101293. [Crossref] [PubMed]
- Mac Aogáin M, Ivan FX, Jaggi TK, et al. Airway "Resistotypes" and Clinical Outcomes in Bronchiectasis. Am J Respir Crit Care Med 2024;210:47-62. [Crossref] [PubMed]
- Mac Aogáin M, Tiew PY, Lim AYH, et al. Distinct "Immunoallertypes" of Disease and High Frequencies of Sensitization in Non-Cystic Fibrosis Bronchiectasis. Am J Respir Crit Care Med 2019;199:842-53. [Crossref] [PubMed]
- Li L, Mac Aogáin M, Xu T, et al. Neisseria species as pathobionts in bronchiectasis. Cell Host Microbe 2022;30:1311-1327.e8. [Crossref] [PubMed]
- Chalmers JD, McHugh BJ, Doherty C, et al. Mannose-binding lectin deficiency and disease severity in non-cystic fibrosis bronchiectasis: a prospective study. Lancet Respir Med 2013;1:224-32. [Crossref] [PubMed]
- Taylor SL, Woodman RJ, Chen AC, et al. FUT2 genotype influences lung function, exacerbation frequency and airway microbiota in non-CF bronchiectasis. Thorax 2017;72:304-10. [Crossref] [PubMed]
- Gao Y, Richardson H, Dicker AJ, et al. Endotypes of Exacerbation in Bronchiectasis: An Observational Cohort Study. Am J Respir Crit Care Med 2024;210:77-86. [Crossref] [PubMed]


