
Combination of radiotherapy and immunochemotherapy improves survival outcomes in non-small cell lung cancer patients with liver metastasis
Highlight box
Key findings
• The combination of radiotherapy and immunochemotherapy provides better survival benefits for non-small cell lung cancer (NSCLC) patients with liver metastasis.
What is known and what is new?
• NSCLC with liver metastasis carries a poor prognosis, and evidence for optimal treatment strategies remains limited. There is limited research on the combination of radiotherapy and immunotherapy for non-small cell lung cancer patients with liver metastasis.
• We start from the Surveillance, Epidemiology, and End Results Program (SEER) database and retrospective data to explore the survival benefits of the combination of immunotherapy and radiotherapy for NSCLC patients with liver metastasis.
What is the implication, and what should change now?
• From our study, it appears that the combination of radiotherapy and immunotherapy offers survival benefits for patients with non-small cell lung cancer and liver metastasis. We should delve deeper into this direction and conduct further prospective research.
Introduction
According to the national cancer report released by the National Cancer Center in 2024, lung cancer is still the leading cause of cancer-related incidence and mortality in China (1). Metastatic non-small cell lung cancer (NSCLC) is a common but refractory subtype, with poor response and prognosis (2). Especially for patients with liver metastasis, the median overall survival (mOS) is only four months, which is the worst compared to other single-organ metastases (3-5). Additionally, the survival rate is negatively associated with the number of liver metastasis (5).
In recent years, immunotherapy represented by immune checkpoint inhibitors (ICIs) such as programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) antibodies has brought a breakthrough for driver gene-negative NSCLC patients and become the first-line treatment for advanced patients. However, for those with liver metastasis, the response rate and survival benefit are limited. Subgroup analysis of two clinical trials, CheckMate 017 and CheckMate 057, showed that the overall survival (OS) in the Nivolumab monotherapy group was 6.8 months, only 1 month longer than that in the chemotherapy group (6). In the study of immunotherapy plus chemotherapy, the subgroup analysis of KEYNOTE-189 showed that Pembrolizumab plus chemotherapy significantly prolonged median OS [12.6 vs. 6.6 months, hazard ratio (HR) =0.62, 95% confidence interval (CI): 0.39–0.98; P<0.001] (7). The subgroup analysis of IMpower 131 showed that Atezolizumab combination chemotherapy had a trend of benefit compared with carboplatin plus albumin paclitaxel in the liver metastasis subgroup (5.5 vs. 4.2 months, HR =0.77, 95% CI: 0.54–1.10) (8). However, in the subgroup of patients with liver metastasis in the IMpower130 study (9), neither OS nor progression-free survival (PFS) was significantly different in the Atezolizumab plus chemotherapy group compared with the chemotherapy group. The same result was also confirmed in the IMpower132 study (10).
From the above studies, we learned that the efficacy of ICIs in NSCLC patients with liver metastasis is unsatisfactory, and there is a significant difference compared to the efficacy in patients with advanced NSCLC without liver metastasis (6). This is possibly due to the specificity of the liver organ, which possesses immune regulatory functions that can maintain local and systemic immune tolerance to self and foreign antigens (11,12). This could explain why patients with liver-metastasized NSCLC have a poorer immune response than patients with NSCLC metastasized to other organs. To address this issue, radiotherapy should be able to leverage its unique advantages. Radiotherapy has always been an essential means of cancer treatment, altering the tumor immune microenvironment (TIME) to transform “cold tumors” into “hot tumors,” enabling the body to generate a better immune response. The combination of radiotherapy and immunotherapy holds more promise for the regression of distant lesions outside the radiation field, known as the “abscopal effect” (13). However, to date, there lacks large targeted cohort studies on the combination of radiotherapy and immunochemotherapy for NSCLC with liver metastasis. Therefore, this study aimed to explore the role of radiotherapy combined with immunochemotherapy in patients with NSCLC and liver metastasis from two aspects: the Surveillance, Epidemiology, and End Results Program (SEER) database (https://seer.cancer.gov/) and the Xinqiao Hospital data. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1977/rc).
Methods
SEER population
The data utilized in this study were obtained from the SEER 17 registry (2000–2021, Nov. 2023 submission), which covered approximately 26.5% of the U.S. population based on 2020 census. Patients diagnosed with NSCLC and liver metastasis were screened. The inclusion criteria were comprised of year of diagnosis between 2010 and 2021, tumor site located in the lung and the bronchus, and one primary site only. Patient age was limited between 18 and 80 years. Patients with reporting source of autopsy only or death certificate only, and those with survival time less or equal to one month were excluded. Furthermore, we excluded patients diagnosed at year 2021 to guarantee at least one year follow-up time.
Study design
In October 24, 2016, the U.S. Food and Drug Administration (FDA) approved pembrolizumab as first-line treatment of advanced NSCLC patients. Therefore, we set 2017 as the boundary. Patients enrolled from 2017 to 2020 received immunochemotherapy as the first-line treatment, and those enrolled from 2010 to 2016 received chemotherapy as the first-line treatment (14,15). Then, based on administration of radiotherapy, patients who received immunochemotherapy were further assigned into two cohorts: immunochemotherapy (IOC cohort) and immunochemotherapy and radiotherapy (RT + IOC cohort). In patients who received chemotherapy, only those also undergoing radiotherapy (CRT cohort) were included. Patients who only underwent chemotherapy in the first-line treatment were not included in this study.
First, survival comparison was conducted between the RT + IOC and IOC cohorts. Univariate and multivariate Cox analyses were performed to identify risk factors affecting survival. Factors with P<0.05 in the univariate analysis were further included in the multivariate analysis. Second, survival comparison was conducted between the RT + IOC and CRT cohorts, including survival analyses both before and after propensity score matching (PSM). Through subgroup analysis, risk factors affecting prognosis were identified. Final, data from single-center was used for comparison (Figure 1).
Covariates in the study included age at diagnosis, sex, race, primary site, laterality, histology, size of the primary tumor, regional lymph node status, and the presence of bone, brain and lung metastases, as well as treatment methods. OS was used as the primary outcome, which was defined as the time interval from diagnosis to death due to any cause. We also analyzed cancer-specific survival (CSS), which was defined as the time interval from diagnosis to death due to cancer. Patients who were alive at the last follow-up or died of other causes were considered censored cases in the survival analysis. The last follow-up time was Dec. 31, 2021.
Xinqiao Hospital data validation
Clinical data of patients diagnosed with NSCLC with liver metastasis who visited Xinqiao Hospital between 2017 and 2024 were screened. Inclusion criteria: (I) patients with NSCLC and liver metastasis confirmed by pathological histology; (II) Eastern Cooperative Oncology Group (ECOG) performance status (16) of 0 or 1; (III) an age of 18–75 years; and (IV) no contraindications to chemotherapy, immunotherapy, or radiotherapy. Exclusion criteria: (I) severe dysfunction of vital organs (heart, liver, and kidneys; biochemical indicators as evaluation criteria); and (II) presence of other malignant tumors. The categories of patients grouping were the same as for SEER data, i.e., RT + IOC cohort, IOC cohort, and CRT cohort. Survival comparisons were then performed between the RT + IOC and IOC cohorts, as well as between the RT + IOC and CRT cohorts, followed by univariate and multivariate analyses to determine risk factors affecting survival.
In the study, covariates included patients’ diagnostic age, gender, smoking status, histology, size of the primary tumor, regional lymph node status, and the presence of bone, brain, and lung metastases, as well as treatment methods. OS was the primary outcome, defined as the time interval from diagnosis to death due to any cause. We also analyzed PFS, defined as the time from the start of treatment until tumor progression or death due to any cause, whichever came first. Patients who were alive or died of other causes at the last follow-up were considered censored cases. The last follow-up time was May 20, 2024. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Institutional Review Board of Xinqiao Hospital (No. 2024-262-01). The SEER database is an open-access database, and no identifiable personal information was involved in the analysis. Therefore, informed consent was waived for this part of the study. However, informed consent was obtained from all patients in Xinqiao Hospital.
Statistical analysis
Baseline clinical characteristics were presented by frequencies and proportion, and were compared using Pearson’s χ2 test. Kaplan-Meier method and log-rank test were used to evaluate survival difference, and Cox proportional hazard model was used to calculate HR with 95% CI. The PSM analysis was performed to match each patient in RT + IOC cohort with two patients in CRT cohort. When performing propensity score matching (PSM), all variables including age at diagnosis, sex, race, primary site, laterality, histology, size of the primary tumor, regional lymph node status, and the presence of bone, brain and lung metastases, as well as treatment methods were considered. All statistical analyses were performed using R software (version 4.4.0; https://www.r-project.org). A two-sided P<0.05 was considered statistically significant.
Results
RT + IOC group vs. IOC group
Baseline
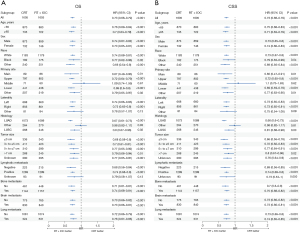
A total of 1,691 patients treated with immunochemotherapy and 1,605 patients with immunochemotherapy and RT were enrolled from SEER database. Compared to the IOC cohort, the RT + IOC cohort had a slightly higher proportion of patients with adenocarcinoma (67.9% vs. 63.4%), while the proportion of those with bone, brain, and lung metastases was significantly higher, at 72.1% vs. 53.3%, 52.3% vs. 14.5%, and 33.1% vs. 26.8%, respectively (Table 1).
Table 1
| Variables | Overall, N=3,296 (%) | RT + IOC, N=1,605 (%) | IOC, N=1,691 (%) | P |
|---|---|---|---|---|
| Age (years) | <0.001 | |||
| <65 | 1,650 (50.1) | 883 (55.0) | 767 (45.4) | |
| ≥65 | 1,646 (49.9) | 722 (45.0) | 924 (54.6) | |
| Sex | 0.87 | |||
| Male | 1,770 (53.7) | 859 (53.5) | 911 (53.9) | |
| Female | 1,526 (46.3) | 746 (46.5) | 780 (46.1) | |
| Race | 0.44 | |||
| White | 2,418 (73.4) | 1,179 (73.5) | 1,239 (73.3) | |
| Black | 380 (11.5) | 175 (10.9) | 205 (12.1) | |
| Other | 498 (15.1) | 251 (15.6) | 247 (14.6) | |
| Primary site | 0.003 | |||
| Main | 168 (5.1) | 86 (5.4) | 82 (4.8) | |
| Upper | 1,562 (47.4) | 802 (50.0) | 760 (44.9) | |
| Middle | 148 (4.5) | 60 (3.7) | 88 (5.2) | |
| Lower | 904 (27.4) | 438 (27.3) | 466 (27.6) | |
| Other | 514 (15.6) | 219 (13.6) | 295 (17.4) | |
| Laterality | 0.04 | |||
| Left | 1,312 (39.8) | 660 (41.1) | 652 (38.6) | |
| Right | 1,778 (53.9) | 861 (53.6) | 917 (54.2) | |
| Other | 206 (6.2) | 84 (5.2) | 122 (7.2) | |
| Histology | 0.03 | |||
| LUAD | 2,161 (65.6) | 1,089 (67.9) | 1,072 (63.4) | |
| LUSC | 548 (16.6) | 246 (15.3) | 302 (17.9) | |
| Other | 587 (17.8) | 270 (16.8) | 317 (18.7) | |
| Tumor size | 0.009 | |||
| ≤3 cm | 717 (21.8) | 343 (21.4) | 374 (22.1) | |
| <3 to ≤5 cm | 836 (25.4) | 423 (26.4) | 413 (24.4) | |
| <5 to ≤7 cm | 571 (17.3) | 299 (18.6) | 272 (16.1) | |
| >7 cm | 488 (14.8) | 245 (15.3) | 243 (14.4) | |
| Unknown | 684 (20.8) | 295 (18.4) | 389 (23.0) | |
| Lymphatic metastasis | 0.001 | |||
| Negative | 494 (15.0) | 215 (13.4) | 279 (16.5) | |
| Positive | 2,579 (78.2) | 1,299 (80.9) | 1,280 (75.7) | |
| Unknown | 223 (6.8) | 91 (5.7) | 132 (7.8) | |
| Bone metastasis | <0.001 | |||
| No | 1,237 (37.5) | 448 (27.9) | 789 (46.7) | |
| Yes | 2,059 (62.5) | 1,157 (72.1) | 902 (53.3) | |
| Brain metastasis | <0.001 | |||
| No | 2,210 (67.1) | 765 (47.7) | 1,445 (85.5) | |
| Yes | 1,086 (32.9) | 840 (52.3) | 246 (14.5) | |
| Lung metastasis | <0.001 | |||
| No | 2,311 (70.1) | 1,074 (66.9) | 1,237 (73.2) | |
| Yes | 985 (29.9) | 531 (33.1) | 454 (26.8) |
IOC, immunochemotherapy; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; RT, radiotherapy; SEER, Surveillance, Epidemiology, and End Results Program.
Survival analysis and Cox analysis
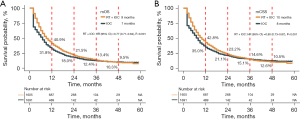
The median OS (mOS) of the RT + IOC group was significantly higher than that of the IOC group (9 vs. 7 months, HR =0.77, 95% CI: 0.71–0.84, P<0.001). In the RT + IOC group, there was a remarkably higher 1-year survival rate and a numerical increase higher 2-year survival rate (Figure 2A). In terms of median CSS (mCSS), the overall results were consistent with mOS. The mCSS was significantly higher in the RT + IOC cohort (10 vs. 8 months, HR =0.80, 95% CI: 0.73–0.87, P<0.001). The 1- and 2-year survival rates were higher in the RT + IOC group (Figure 2B). Univariate and multivariate Cox analyses revealed that female patients, other races except White and Black, and radiotherapy combined with immunochemotherapy treatment were common protective factors for OS and CSS. Black race, NSCLC other than adenocarcinoma and tumors larger than 7 cm were common risk factors for OS and CSS. In addition, an age above 65 years was a risk factor for OS (Table S1).
Xinqiao Hospital data validation
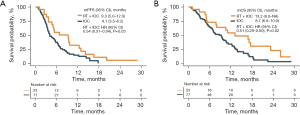
A total of 77 patients in IOC cohort and 23 patients in RT + IOC cohort were enrolled from Xinqiao Hospital. There were no significant differences in the baseline characteristics between the two groups (Table 2). Survival analysis revealed that the median progression-free survival (mPFS) of the RT + IOC group was significantly higher than that of the IOC group (9.3 vs. 4.1 months, HR =0.54, 95% CI: 0.31–0.94, P=0.03) (Figure 3A), as well as a significantly higher mOS in the RT + IOC group (13.2 vs. 8.7 months, HR =0.51, 95% CI: 0.29–0.90, P=0.02) (Figure 3B). The Cox analyses found that, after adjusting, RT combined with IOC treatment remained an independent protective factor for both PFS and OS (Table S2).
Table 2
| Variables | Overall, N=100 (%) | RT + IOC, N=23 (%) | IOC, N=77 (%) | P |
|---|---|---|---|---|
| Age (years) | 0.91 | |||
| <65 | 62 (62.0) | 15 (65.2) | 47 (61.0) | |
| ≥65 | 38 (38.0) | 8 (34.8) | 30 (39.0) | |
| Sex | 0.99 | |||
| Male | 84 (84.0) | 19 (82.6) | 65 (84.4) | |
| Female | 16 (16.0) | 4 (17.4) | 12 (15.6) | |
| Histology | 0.35 | |||
| LUSC | 41 (41.0) | 7 (30.4) | 34 (44.2) | |
| Non-LUSC | 59 (59.0) | 16 (69.6) | 43 (55.8) | |
| Smoking | 0.18 | |||
| Never | 30 (30.0) | 10 (43.5) | 20 (26.0) | |
| Current or former | 70 (70.0) | 13 (56.5) | 57 (74.0) | |
| Tumor size | 0.31 | |||
| ≤3 cm | 30 (30.0) | 8 (34.8) | 22 (28.6) | |
| <3 to ≤5 cm | 23 (23.0) | 6 (26.1) | 17 (22.1) | |
| <5 to ≤7 cm | 19 (19.0) | 3 (13.0) | 16 (20.8) | |
| >7 cm | 25 (25.0) | 4 (17.4) | 21 (27.3) | |
| Unknown | 3 (3.0) | 2 (8.7) | 1 (1.3) | |
| Lymphatic metastasis | 0.26 | |||
| Negative | 20 (20.0) | 7 (30.4) | 13 (16.9) | |
| Positive | 80 (80.0) | 16 (69.6) | 64 (83.1) | |
| Bone metastasis | 0.07 | |||
| No | 45 (45.0) | 6 (26.1) | 39 (50.6) | |
| Yes | 55 (55.0) | 17 (73.9) | 38 (49.4) | |
| Brain metastasis | 0.31 | |||
| No | 83 (83.0) | 17 (73.9) | 66 (85.7) | |
| Yes | 17 (17.0) | 6 (26.1) | 11 (14.3) | |
| Lung metastasis | 0.17 | |||
| No | 58 (58.0) | 10 (43.5) | 48 (62.3) | |
| Yes | 42 (42.0) | 13 (56.5) | 29 (37.7) |
IOC, immunochemotherapy; LUSC, lung squamous cell carcinoma; RT, radiotherapy.
RT + IOC group vs. CRT group
Baseline characteristics and survival analysis before and after PSM
Between 2010 and 2016, 3,013 patients with confirmed NSCLC and liver metastasis who underwent both chemotherapy and radiotherapy were extracted from the SEER database (CRT group), and were compared with the aforementioned RT + IOC group. The comparison of clinical characteristics revealed that among patients with NSCLC liver metastasis, 76.4% were Caucasian and 63.9% had adenocarcinoma. The characteristics with significant differences at baseline between the two groups of patients included diagnostic age, race, histology, lymph node metastasis, and bone metastasis (Table 3).
Table 3
| Variables | Before PSM | After PSM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall, N=4,618 (%) |
RT + IOC, N=1,605 (%) |
CRT, N=3,013 (%) |
P | Overall, N=3,210 (%) |
RT + IOC, N=1,605 (%) |
CRT, N=1,605 (%) |
P | ||||||||
| Age (years) | 0.01 | 0.67 | |||||||||||||
| <65 | 2,661 (57.6) | 883 (55.0) | 1,778 (59.0) | 1,753 (54.6) | 883 (55.0) | 870 (54.2) | |||||||||
| ≥65 | 1,957 (42.4) | 722 (45.0) | 1,235 (41.0) | 1,457 (45.4) | 722 (45.0) | 735 (45.8) | |||||||||
| Sex | 0.78 | 0.65 | |||||||||||||
| Male | 2,486 (53.8) | 859 (53.5) | 1,627 (54.0) | 1,732 (54.0) | 859 (53.5) | 873 (54.4) | |||||||||
| Female | 2,132 (46.2) | 746 (46.5) | 1,386 (46.0) | 1,478 (46.0) | 746 (46.5) | 732 (45.6) | |||||||||
| Race | <0.001 | 0.82 | |||||||||||||
| White | 3,527 (76.4) | 1,179 (73.5) | 2,348 (77.9) | 2,362 (73.6) | 1,179 (73.5) | 1,183 (73.7) | |||||||||
| Black | 516 (11.2) | 175 (10.9) | 341 (11.3) | 357 (11.1) | 175 (10.9) | 182 (11.3) | |||||||||
| Other | 575 (12.5) | 251 (15.6) | 324 (10.8) | 491 (15.3) | 251 (15.6) | 240 (15.0) | |||||||||
| Primary site | 0.14 | 0.86 | |||||||||||||
| Main | 258 (5.6) | 86 (5.4) | 172 (5.7) | 175 (5.5) | 86 (5.4) | 89 (5.5) | |||||||||
| Upper | 2,307 (50.0) | 802 (50.0) | 1,505 (50.0) | 1,599 (49.8) | 802 (50.0) | 797 (49.7) | |||||||||
| Middle | 194 (4.2) | 60 (3.7) | 134 (4.4) | 131 (4.1) | 60 (3.7) | 71 (4.4) | |||||||||
| Lower | 1,176 (25.5) | 438 (27.3) | 738 (24.5) | 879 (27.4) | 438 (27.3) | 441 (27.5) | |||||||||
| Other | 683 (14.8) | 219 (13.6) | 464 (15.4) | 426 (13.3) | 219 (13.6) | 207 (12.9) | |||||||||
| Laterality | 0.15 | 0.94 | |||||||||||||
| Left | 1,815 (39.3) | 660 (41.1) | 1,155 (38.3) | 1,328 (41.4) | 660 (41.1) | 668 (41.6) | |||||||||
| Right | 2,540 (55.0) | 861 (53.6) | 1,679 (55.7) | 1,717 (53.5) | 861 (53.6) | 856 (53.3) | |||||||||
| Other | 263 (5.7) | 84 (5.2) | 179 (5.9) | 165 (5.1) | 84 (5.2) | 81 (5.0) | |||||||||
| Histology | <0.001 | 0.57 | |||||||||||||
| LUAD | 2,953 (63.9) | 1,089 (67.9) | 1,864 (61.9) | 2,162 (67.4) | 1,089 (67.9) | 1,073 (66.9) | |||||||||
| LUSC | 763 (16.5) | 246 (15.3) | 517 (17.2) | 514 (16.0) | 246 (15.3) | 268 (16.7) | |||||||||
| Other | 902 (19.5) | 270 (16.8) | 632 (21.0) | 534 (16.6) | 270 (16.8) | 264 (16.4) | |||||||||
| Tumor size | 0.54 | 0.76 | |||||||||||||
| ≤3 cm | 954 (20.7) | 343 (21.4) | 611 (20.3) | 679 (21.2) | 343 (21.4) | 336 (20.9) | |||||||||
| <3 to ≤5 cm | 1,193 (25.8) | 423 (26.4) | 770 (25.6) | 834 (26.0) | 423 (26.4) | 411 (25.6) | |||||||||
| <5 to ≤7 cm | 848 (18.4) | 299 (18.6) | 549 (18.2) | 609 (19.0) | 299 (18.6) | 310 (19.3) | |||||||||
| >7 cm | 715 (15.5) | 245 (15.3) | 470 (15.6) | 513 (16.0) | 245 (15.3) | 268 (16.7) | |||||||||
| Unknown | 908 (19.7) | 295 (18.4) | 613 (20.3) | 575 (17.9) | 295 (18.4) | 280 (17.4) | |||||||||
| Lymphatic metastasis | 0.008 | 0.91 | |||||||||||||
| Negative | 599 (13.0) | 215 (13.4) | 384 (12.7) | 438 (13.6) | 215 (13.4) | 223 (13.9) | |||||||||
| Positive | 3,814 (82.6) | 1,299 (80.9) | 2,515 (83.5) | 2,588 (80.6) | 1,299 (80.9) | 1,289 (80.3) | |||||||||
| Unknown | 205 (4.4) | 91 (5.7) | 114 (3.8) | 184 (5.7) | 91 (5.7) | 93 (5.8) | |||||||||
| Bone metastasis | 0.001 | 0.64 | |||||||||||||
| No | 1,437 (31.1) | 448 (27.9) | 989 (32.8) | 909 (28.3) | 448 (27.9) | 461 (28.7) | |||||||||
| Yes | 3,181 (68.9) | 1,157 (72.1) | 2,024 (67.2) | 2,301 (71.7) | 1,157 (72.1) | 1,144 (71.3) | |||||||||
| Brain metastasis | 0.09 | 0.75 | |||||||||||||
| No | 2,281 (49.4) | 765 (47.7) | 1,516 (50.3) | 1,540 (48.0) | 765 (47.7) | 775 (48.3) | |||||||||
| Yes | 2,337 (50.6) | 840 (52.3) | 1,497 (49.7) | 1,670 (52.0) | 840 (52.3) | 830 (51.7) | |||||||||
| Lung metastasis | 0.98 | 0.82 | |||||||||||||
| No | 3,093 (67.0) | 1,074 (66.9) | 2,019 (67.0) | 2,155 (67.1) | 1,074 (66.9) | 1,081 (67.4) | |||||||||
| Yes | 1,525 (33.0) | 531 (33.1) | 994 (33.0) | 1,055 (32.9) | 531 (33.1) | 524 (32.6) | |||||||||
CRT, chemotherapy + radiotherapy; IOC, immunochemotherapy; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PSM, propensity score matching; RT, radiotherapy; SEER, Surveillance, Epidemiology, and End Results Program.
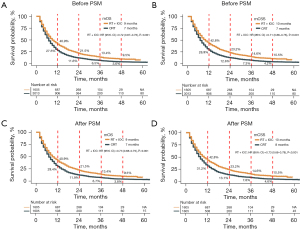
Before PSM, the mOS and mCSS of patients in the RT + IOC group were significantly higher than those in the CRT group, and the survival rates from 1 to 4 years were all higher than those in the CRT group (Figure 4A,4B). To eliminate the bias caused by baseline inconsistencies, we adopted a 1:1 PSM analysis to balance the baseline characteristics (Figure S1). No significant difference between the two cohorts was found after matching (Table 3). After PSM, the mOS of patients in the RT + IOC group was still significantly higher than that in the CRT group (9 vs. 7 months, HR =0.73, 95% CI: 0.68–0.79, P<0.001), and the survival rates from 1 to 4 years were all higher than those in the CRT group (Figure 4C). The results of the mCSS indicator were consistent with mOS, since the mCSS of patients was significantly higher in the RT + IOC group than in the CRT group (10 vs. 8 months, HR =0.73, 95% CI: 0.68–0.79, P<0.001), and the survival rates from 1 to 4 years were all higher than those in the CRT group (Figure 4D).
Subgroup analysis
To further explore the factors affecting the prognosis of OS and CSS, we conducted a subgroup analysis based on the matched patients (Figure 5). As shown in the forest plot, regardless of the impact on OS or CSS, in almost all subgroups, the RT + IOC group had significant survival benefits. Interestingly, no significant difference was observed in the combined treatment group between other histology types (non-squamous and non-adenocarcinoma NSCLC).
Xinqiao Hospital data validation
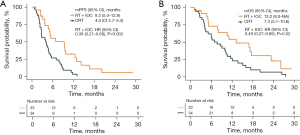
To further validate the survival difference between the RT + IOC group and the CRT group, we retrospectively collected 34 NSCLC patients with liver metastasis who received both chemotherapy and radiotherapy at Xinqiao Hospital (CRT group), and compared them with the RT + IOC group (23 cases). There were no significant differences in the baseline characteristics between the two groups (Table S3). The mPFS of the RT + IOC group was significantly higher than that of the CRT group (9.3 vs. 4.0 months, HR =0.38, 95% CI: 0.21–0.69, P=0.002) (Figure 6A), and the mOS of the RT + IOC group was significantly higher than that of the CRT group (13.2 vs. 7.3 months, HR =0.49, 95% CI: 0.27–0.89, P=0.02) (Figure 6B). In the univariate analysis of PFS and OS, only the factor of combined RT + IOC treatment showed a significant difference and was a protective factor for PFS and OS (Table S4).
Discussion
In the studies of PACIFIC trial and Theelen et al., the combined use of immunotherapy and radiotherapy achieved great success in patients with locally advanced and metastatic NSCLC, significantly improving PFS and OS, reflecting the radiotherapy’s role in promoting systemic immunity (17,18). The PACIFIC-5 trial also demonstrated similar results (19). However, the NRG-LU002(NCT03137771) trial (20) indicated that incorporating local consolidative therapy (LCT) into immunotherapy did not significantly enhance PFS or OS for patients with oligometastatic NSCLC. The recently announced results of the PACIFIC-2 (NCT03519971) trial (21) showed that, compared with the control group, the combination of radiotherapy and immunotherapy resulted in a 4.4-month improvement in PFS (no statistically significant differences). In a series of studies from the PACIFIC trials, considering that the radiotherapy sites were mostly located in the lungs and the purpose of the radiotherapy was curative, these factors may lead to different immune responses and side effects, which in turn may further impact survival outcomes. Yu et al. found that liver metastasis can induce acquired immune resistance in the body, but stereotactic body radiotherapy (SBRT) can reshape the liver immune microenvironment, thereby promoting systemic antitumor immune responses (22). Thus, it is evident that the synergistic effect of radiotherapy and immunotherapy is still a subject of controversy. Therefore, targeting the specific population of NSCLC patients with liver metastasis, and without restricting the site of their radiotherapy, we use data from both the SEER database and a single-center dataset to cross-validate and further investigate this issue. In our study, the radiation dose, irradiation site, target volume, and treatment purpose all differ from those in the aforementioned studies. This has resulted in a relatively lower risk associated with the combination of radiotherapy and immunotherapy in our research. The final results indicate that both databases indicated that the treatment combining radiotherapy with immunochemotherapy was significantly superior to the other groups, providing better survival benefits for such patients.
Although our study results indicate that the combination of radiotherapy and immunotherapy can achieve better therapeutic effects, further research is still needed on how radiotherapy and immunotherapy can better exert their synergistic effects. The fractionation pattern of radiotherapy may be an important factor in this regard. Generally, large-fraction radiotherapy can induce tumor cell death and release antigens, producing an “in situ vaccine” effect, and activating the immune system (13). In the study by Welsh et al. (23), high-dose large-fraction SBRT (50 Gy/4 fractions) combined with PD-1 inhibitors showed good efficacy and safety in NSCLC. A basic study by Yin et al. (24) found that the triple therapy model of primary tumor large-fraction radiotherapy + distant tumor low-dose radiotherapy + immune checkpoint inhibitors achieved the best distant tumor control rate. The principle is that large-fraction radiotherapy induces apoptosis of in situ tumor cells, exposing tumor-specific antigens and sensitizing tumor-specific T cells; while low-dose radiotherapy promotes the migration of tumor-specific T cells to distant tumors, regulating the immune microenvironment of distant tumors, and the combination of the two therapies produces a CD8+ T cell-dependent immune effect; finally, the tumor-killing activity of T cells is restored through PD-1 inhibitors, further enhancing systemic anti-tumor effects. Spatial fractionated radiotherapy (SFRT) is an emerging radiotherapy technology that irradiates large-volume primary or metastatic malignant tumors by creating highly heterogeneous dose distributions in three-dimensional space. Due to the different damages caused by the dose or spatial position of the beam, the peak-valley distribution of SFRT may induce unique systemic effects, which may better activate the immune system (25). There have been case reports on the efficacy of SFRT combined with immunotherapy (26,27), but more clinical evidence is needed to confirm its safety and feasibility.
In the survival analysis of the RT + IOC group versus the IOC group across the two databases, the baseline comparison between the two groups showed that patients in the RT + IOC group had higher rates of bone, brain, and lung metastases. Although there was no statistical difference between the two groups, the baseline status of patients in the RT + IOC group was relatively worse. However, interestingly, patients in the RT + IOC group had better OS and CSS. This further illustrates that the combination of radiotherapy and immunotherapy plays a significant role. In these two groups, although the combined treatment group had better median mOS, mCSS and 1- and 2-year survival rate, the 3- and 4-year survival rates were similar. This finding indicates that the method of radiotherapy combined with immunochemotherapy does not bring long-term survival benefits to such patients. This difference is probably due to different tumor burdens, as the immune activation effect brought by radiotherapy + immunochemotherapy may not be sufficiently significant for patients with a large tumor burden. However, due to the limited number of cases that met the criteria of this study in real-world research, further subgroup analysis cannot be conducted for verification. In addition to the aforementioned shortcomings, the limited information in the SEER database prevented us from determining the site, dose, fractionation method, and the sequence of systemic therapy and radiotherapy, molecular data, the number of metastatic sites per patient, and toxicity assessments, thus we cannot determine the optimal combination of radiotherapy and immunotherapy. While using the year 2017 as a demarcation for immunotherapy has some basis, it may still lead to biased results. In order to ensure the rigor of our research, however, we have strictly controlled the inclusion and exclusion criteria. As a result, the number of patients enrolled in the study is relatively small. Thus, we could not use the site of radiotherapy and fractionation method as covariates for subgroup analysis. Therefore, our exploration of radiotherapy combined with immunochemotherapy is not deep enough. To further clarify whether the fractionation method of radiotherapy, the site of radiotherapy, and the number of target lesions covered by the radiation field will affect the efficacy of combined immunotherapy for patients with NSCLC with liver metastasis, large-scale prospective studies are necessary for further verification.
Conclusions
The combination of radiotherapy and immunochemotherapy provides better survival benefits for NSCLC patients with liver metastasis than immunochemotherapy alone or chemotherapy + radiotherapy. Further research is needed to explore the optimal radiotherapy method for this patient population.
Acknowledgments
We would like to thank the efforts of the SEER program tumor registries in the creation of the SEER database.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1977/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1977/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1977/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-1977/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Institutional Review Board of Xinqiao Hospital (No. 2024-262-01). The SEER database is an open-access database, and no identifiable personal information was involved in the analysis. Therefore, informed consent was waived for this part of the study. However, informed consent was obtained from all patients in Xinqiao Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Yoon JY, Sigel K, Martin J, et al. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J Thorac Oncol 2019;14:184-92. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Tang C, Liao Z, Hess K, et al. Prognosis and predictors of site of first metastasis after definitive radiation therapy for non-small cell lung cancer. Acta Oncol 2016;55:1022-8. [Crossref] [PubMed]
- Kitadai R, Okuma Y, Hakozaki T, et al. The efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer with liver metastases. J Cancer Res Clin Oncol 2020;146:777-85. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol 2020;15:1351-60. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Nishio M, Barlesi F, West H, et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J Thorac Oncol 2021;16:653-64. [Crossref] [PubMed]
- Lee JC, Mehdizadeh S, Smith J, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 2020;5:eaba0759. [Crossref] [PubMed]
- Lindblad KE, Lujambio A. Liver metastases inhibit immunotherapy efficacy. Nat Med 2021;27:25-7. [Crossref] [PubMed]
- Donlon NE, Power R, Hayes C, et al. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett 2021;502:84-96. [Crossref] [PubMed]
- Xie L, Zhang Z. Survival benefit of combined immunotherapy and chemoradiotherapy in locally advanced unresectable esophageal cancer: an analysis based on the SEER database. Front Immunol 2024;15:1334992. [Crossref] [PubMed]
- Ma JC, Zhang JX, Wang F, et al. The Effect of immunotherapy on oligometastatic non-small cell lung cancer patients by sites of metastasis. Front Immunol 2022;13:1039157. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467-75. [Crossref] [PubMed]
- Wu YL, Wu L, Bi N, et al. PACIFIC-5: A phase III study of consolidation durvalumab (D) in patients (pts) with unresectable stage III NSCLC and no progression after concurrent or sequential chemoradiotherapy (cCRT or sCRT). Ann Oncol 2024;35:S1624.
- Iyengar P, Hu C, Gomez DR, et al. NRG-LU002: Randomized phase II/III trial of maintenance systemic therapy versus local consolidative therapy (LCT) plus maintenance systemic therapy for limited metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2024;42:8506.
- Bradley JD, Nishio M, Okamoto I, et al. PACIFIC-2: Phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. 2024 ELCC abstrLBA1.
- Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152-64. [Crossref] [PubMed]
- Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 2020;8:e001001.
- Yin L, Xue J, Li R, et al. Effect of Low-Dose Radiation Therapy on Abscopal Responses to Hypofractionated Radiation Therapy and Anti-PD1 in Mice and Patients With Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2020;108:212-24. [Crossref] [PubMed]
- Johnsrud AJ, Jenkins SV, Jamshidi-Parsian A, et al. Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations. Radiat Res 2020;194:688-97. [Crossref] [PubMed]
- Jiang L, Li X, Zhang J, et al. Combined High-Dose LATTICE Radiation Therapy and Immune Checkpoint Blockade for Advanced Bulky Tumors: The Concept and a Case Report. Front Oncol 2020;10:548132. [Crossref] [PubMed]
- Massaccesi M, Boldrini L, Romano A, et al. Unconventional radiotherapy to enhance immunotherapy efficacy in bulky tumors: a case report. Immunotherapy 2021;13:1457-63. [Crossref] [PubMed]


