Shifted focus of bronchoalveolar lavage in patients with suspected thoracic malignancy: an analysis of 224 patients
Introduction
Bronchoscopy has been an integral part of diagnosis and staging for patients suspected with thoracic malignancies. Interventional pulmonologists have the options of combining different techniques to obtain tissue samples and to maximize diagnostic yields. However, keeping bronchoscopic evaluation streamlined, which involves reducing redundancy and having each approach serving the best purpose, will have great implications in terms of efficiency and cost.
Bronchoalveolar lavage (BAL) cytology constitutes a standard part in bronchoscopic evaluation for thoracic malignancies, which is primarily supported by earlier studies before introduction of image-guided navigation, showing 28% to 65% diagnostic yield for malignant peripheral lung lesions with BAL (1-3). However, major lung cancer guidelines currently recommend determination of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) status for all non-squamous cell types in non-small cell lung cancer (NSCLC) (4,5), and shifting treatment paradigm of lung cancer and other malignancies relies heavily on a growing list of predictive and prognostic biomarkers (6-10). Subtyping and genotyping tests would require sufficient tumor tissues which are usually more than that could be obtained from BAL. But whether omitting BAL cytology would compromise cancer diagnosis remains unknown and further evidence is needed if we had to modify our practice.
Co-infection in patients with thoracic malignancies is common. For infectious disease, BAL has the advantage of a larger sampling volume. However, when BAL culture would be indicated for patients primarily suspected with malignancy remains to be determined. We aim to assess the role of BAL in patients with suspected thoracic malignancies. We hypothesize that in context of comprehensive sampling with transbronchial brushings (TBB), transbronchial lung biopsies (TBLBx) and transbronchial needle aspirations (TBNA), BAL might not be additive to cancer diagnosis, while how to set threshold for BAL culture in this population remains to be explored.
Methods
Patient inclusion
We retrospectively analyzed a prospectively kept bronchoscopy database for patients with suspected thoracic malignancies at the Johns Hopkins Hospital. Patients were excluded if they had previous hematological malignancies, or were immunocompromised (received bone marrow/solid organ transplantation, with hereditary/acquired/iatrogenic immunodeficiency). The Institutional Review Board at the Johns Hopkins Medical Institute approved this study (JHMI IRB NA_00026855). Consents were obtained from all patients.
For included patients, clinical characteristics, characteristics of thoracic imaging [thoracic computer-tomographic (CT) or whole-body positron emission tomography (PET) scan], results of index bronchoscopy and BAL cytology/culture were recorded. Patients were followed up if the index bronchoscopy failed to establish a definitive diagnosis of cancer. A definitive benign etiology was confirmed by specific pathology or by radiographically stable lesion over 2 years or by spontaneous lesion shrinkage without anti-tumor therapy.
The pre-test suspicion for underlying or coexisting pulmonary infection was independently evaluated by two pulmonologists (Xin Zhang and Rex C. Yung) for each case. Disparities were referred to a third physician (Yuan Zhang) for final judgement. Clinical suspicion for lower respiratory tract infection (LRTI) is based on one or more of following symptoms/signs: Body temperature >37.5 °C within 48 h of inclusion, leukocytosis (>10×109/L), newly developed or worsening cough, sputum purulence, dyspnea (11).
BAL culture for infectious pathogens was classified into three categories (primary infection, colonization/possible infection, negative) based on established criteria for each pathogen group (12). Primary infection was diagnosed with positive bacteriology results in BAL (i.e., culture yielding a single pathogenic bacterial microorganism at the minimum concentration of 103 cfu/mL or any microorganism excluding mouth flora above the minimum concentration of 104 cfu/mL; or the identification of Legionella spp., Nocardia spp., Chlamydia spp., Mycoplasmas, Mycobacteria, Aspergillus spp., Cryptococcus spp., Pneumocystis jirovecii regardless of colony counts). Bacterial cultures fewer than 103 cfu/mL were considered as colonization/possible infection.
Bronchoscopy sampling strategy
Flexible bronchoscopy was performed with the patient under conscious sedation using fentanyl and midazolam according to the British Thoracic Society guidelines (13). BAL was routinely performed in all patients undergoing diagnostic bronchoscopy for suspected thoracic malignancy, and was performed by three installations of 50 mL sterile saline over the working channel of the bronchoscope and was recovered by suction according to standard guidelines and as described earlier (14-16). In patients with diffuse pulmonary infiltrates or with solely mediastinal/hilar lymphadenopathy (BAL indicated to rule out endotracheal spread of disease and infection), BAL was performed either in the right middle lobe or the lingula. For patients with focal lesions, BAL was performed in corresponding pulmonary segment. The choice of further sampling techniques, combinations of endobronchial/transbronchial forceps biopsies, TBNA with or without endobronchial ultrasound (EBUS), and endobronchial/TBB was at the pulmonologist’s discretion. Often multiple sites were sampled and multiple techniques used to obtain sufficient sample for subtyping, genotyping and staging when indicated. BAL was universally sent for bacteria culture, while evaluation for mycobacterium, fungus, and virus was performed when clinically indicated.
Statistical analysis
Statistical analyses were done with Stata version 12 (StataCorp LP, College Station, TX, USA). Group differences were examined using Chi-square test. We investigated possible demographic, clinical and radiographic predictive factors for BAL to detect primary LRTI in patients primarily suspected for lung malignancy. Univariate associations for the outcome (positive or negative primary infection) were investigated with logistic regression, adjusted for age. We included variables with P≤0.20 in multivariable analysis using backward elimination process. Variables with P≤0.05 (two tails) in multivariable analysis were retained in the final model.
Results
Demographics of included patients
From November 2009 to May 2013, 224 patients were included. Figure 1 details the patient flow and diagnosis information.
Clinical characteristics of included patients are summarized in Table 1. Median age was 63 years (range, 19–94 years); 55.4% were male; 23.7% were current and 53.1% were former smokers. 63.4% had history of solid malignancy, and correspondingly 41.5% were referred for bronchoscopy because of positive follow-up imaging while remained asymptomatic.

Full table
Yield by BAL compared to overall bronchoscopy for diagnosis of malignancies
Among 224 patients, 156 were diagnosed as malignant on index bronchoscopy (Table 1), of which 85 were with NSCLC [51 adenocarcinomas, 29 squamous cell carcinomas, 5 non-small cell lung cancer-not otherwise specified (NSCLC-NOS)]; 13 were with small cell lung cancer (SCLC); 4 were with carcinoid; 50 were with metastasis from extra-thoracic cancers and 4 were with primary parenchymal lung lymphomas. Of the remaining 68 patients, 23 were diagnosed with malignancy on follow-up, giving an 87.2% (156/179) initial diagnostic yield for malignancy. 40 cases were confirmed as benign (Figure 1).
Patients were categorized into six groups based on chest radiographic features (Table 2). By BAL alone the diagnostic yields were 50% for peripheral consolidations, and were lower with smaller and more peripheral lesions, being 21.4% for masses >3 cm, and 7.1% for small peripheral nodules <2 cm. No cancer was diagnosed solely by BAL cytology. Combining all techniques, overall diagnostic yield for malignant lesions ranged from 66.7% (small nodules <2 cm) to 100% (peripheral consolidations). Table 2 outlines the comprehensive tissue sampling strategy adopted.
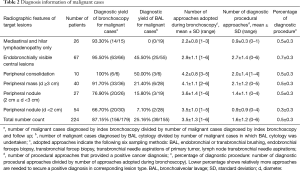
Full table
BAL in the diagnosis of underlying or coexisting LRTI
All 224 patients had BAL for bacteria culture, of which 30 had primary LRTIs. One hundred seventy-three patients had BAL for mycobacteria culture, of which 5 were positive. Two hundred and five patients had BAL for fungal culture, of which 12 were primary infections. Seventy two patients had BAL for viral cultures or PCR test, none of which reported positive. Overall, 41 cases were classified as primary LRTIs, of which 33 were also diagnosed with a thoracic malignancy, 1 with concurrent sarcoidosis. Table 3 lists the pathogens detected in LRTI patients.
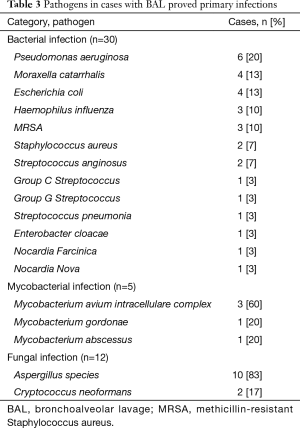
Full table
Table 4 details the breakdown of patients by pre-test suspicion for infections and BAL culture results. Twenty patients (48.8% in the primary infection group) were proved with LRTI by BAL even without clinical indication for infections. Proportions of patients with different infectious status do not differ significantly between patients with and without pre-test suspicion for infections (P=0.199), suggesting common practice to perform BAL culture in selected patients based on clinical suspicion could lead to underdiagnoses of infection.

Full table
Patients’ risk factors for BAL to detect primary LRTI
We studied 23 demographic, clinical and radiographic variables as potential predictors for BAL to detect primary LRTI in this population with primary concern for thoracic malignancy. Age ≥75 years, coexisting chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM) and white blood cell count <4,000/mL were significantly associated with BAL proved primary LRTI by univariate analysis (Table 5). Since blood counts were missing in 39 patients during peri-procedure period, we couldn’t include this parameter in multivariate model. Variables with P≤0.2 were retained for multivariable logistic regression, which indicated age ≥75 years, coexisting COPD or DM significantly increased the likelihood of BAL detected LRTI by 3.0 fold (OR 3.0; 95% CI: 1.29–7.06), 2.7 fold (adjusted OR 2.7; 95% CI: 1.14–6.26), and 4.5 fold (adjusted OR 4.5; 95% CI: 1.90–10.44), respectively. There was no significant association between primary infection and pulmonary symptoms, radiographic infiltration, concurrent systemic steroids/immunosuppressant therapy or recent chemotherapy in our study population.
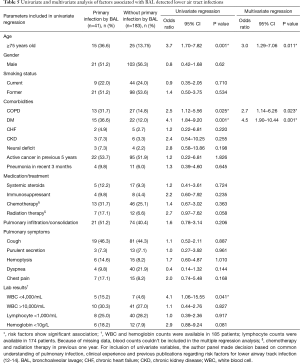
Full table
Discussion
This study reevaluated a routinely adopted bronchoscopic approach in the context of comprehensive diagnostic strategy. We have shown that diagnostic yield of BAL for malignancy was highest at 50% (3/6) with cases presenting as peripheral consolidations, and decreased to the lowest 7.1% (2/28) in patients with small peripheral nodules (diameter <2 cm). Overall, diagnostic yields of BAL comprise only 10.6% (small peripheral nodules) to 50% (peripheral consolidations) of the cumulative bronchoscopy yields in our cohort, and no cancer was diagnosed solely by BAL cytology. We also showed that BAL identified potentially dangerous yet treatable infections in patients primarily suspected for thoracic malignancy (18.3% in our cohort), and 48.8% of those would have been missed if BAL culture had only been performed based on clinical suspicion for infections. Taken together, our results advocate not depending on BAL cytology for cancer diagnosis while lowering the threshold for BAL culture in patients primarily suspected for thoracic malignancy. Considering the large number of patients that would require bronchoscopies for cancer evaluation, this modification could have significant practical implications in terms of diagnostic accuracy, cost and efficiency.
BAL is currently a routine practice in bronchoscopic evaluation for patients with suspected thoracic malignancies largely because previous studies proved it as a useful tool in diagnosing peripheral lesions (17). However, three ongoing changes prompted us to make a new evaluation. Firstly, smaller and more peripheral lesions are more often presented for bronchoscopic evaluation, for which our study showed BAL is the least helpful. With major organizations recommending screening with yearly low-dose CT for selected high-risk current and former smokers (18-22), more positive screening would need diagnostic assessment. Also, routine image follow-up for an enlarging cancer survivor population would pick up more asymptomatic, hence much smaller lesions. 41.5% of our included patients were referred due to positive image results reflected this trend. Secondly, shifting therapeutic paradigm for lung cancer and other malignancies would require adequate tissue samples to support cancer subtyping, genotyping and staging. A positive cytology from BAL alone would fall short on these requirements, making personalized targeted treatment impossible. Thirdly, the above challenges are coupled with advances in bronchoscopy instruments and image-guiding interventions. Planar and CT-fluoroscopy (23), EBUS (24-26) and virtual bronchoscopic navigation (27-29) have improved targeting for endoscopically non-visible lesions. Current NCCN guideline recommends radial EBUS or navigational bronchoscopy as the preferred biopsy approach for peripheral lesions (5,30). Once directed to a target lesion, TBLBx and TBNA are expected to play a more important role since they would garner larger amount of tissue. BAL is generally safe but is not without risks. BAL has a reported complication rate of 0–49%, depending on how the complications were defined, and deaths associated with BAL have been reported (31-34). The main complications associated with BAL are desaturation, a drop in FEV1 and hemorrhage.
The diagnostic yield of BAL in our study appears lower than that in previous reports. Poletti et al. reported a 76% diagnostic yield of BAL for overall malignant cases (35). However, previous studies tend to include patients with more diffuse lesions and more advanced diseases (27.2% with bronchoalveolar carcinoma type, 42.6% with carcinomatous lymphangitis and 13.6% with hematogenous metastatic disease). Our diagnostic yields of the overall bronchoscopies are comparable with studies adopting guided bronchoscopies (66.7–94.7% for peripheral lesions >2 cm, and 18.2–77.8% for lesions ≤2 cm) (29,36-39), and contrasted with those from non-guided bronchoscopies (Baaklini et al. reported diagnostic yields of 14% and 31% for lesions ≤2 cm when located in the peripheral one third vs. the inner two thirds of the lung) (40). Hence our results remain representative of current practice of guided bronchoscopy.
In our study, the percentage of NSCLC-NOS among NSCLC is 5.9%, which is much lower compared to around 20% reported in recent series (41,42). Overall survival for NSCLC-NOS patients was lowest among patients with NSCLC, and it has been shown that relying on cytology alone were associated with higher risk for a diagnosis of NSCLC-NOS (41). We attribute our low NSCLC-NOS proportion to the consistent adoption of comprehensive sampling strategy.
Co-infection in patients with bronchogenic carcinoma are common (43-46), yet there is no consensus as to where to set the threshold for BAL culture. Practice varies between institutions and physicians and is largely based on clinical suspicion. We found 18.3% patients had BAL proved LRTI even the primary suspicion were malignancy. Notably clinical suspicion doesn’t effectively separate patients with and without primary LRTI. Further investigation of the pathogens suggests these would cause severe outcomes if untreated, especially for patients who would be immunosuppressed from cancer treatment. It’s not safe to restrain BAL culture only in patients with clinical suspicion for infections, which is unfortunately a common practice.
We showed that age ≥75, coexisting COPD or DM were associated with significantly increased BAL proven LRTI. Reasoning would suggest factors like recent chemotherapy, consolidation on image should be risk factors for pulmonary infection. The fact that those variables didn’t show significant association reflects our selection of study population, which excluded patients whose primary concerns were infectious etiology only.
Our study had several limitations. Bronchoscopies in this study were performed by experienced interventional pulmonologists with assistance of appropriate imaging guiding; this could represent a higher standard of performance than average bronchoscopy services. However, the fact that the diagnostic yield could be achieved consistently and falls in the previously reported range indicate that the comprehensive sampling approach could be repeated and would do patients great benefit by avoiding repetitive procedures and reducing risks of being labelled as NSCLC-NOS. We did not study how the BAL cultures have influenced clinical care for included patients, since the majority of which were treated on outpatient basis by different physicians. Whether BAL-directed anti-microbial therapy would be beneficial remains to be explored.
When new techniques are increasingly integrated into routine diagnostic workup, the decision to discard or modify the traditional technique should be based on solid evidence to avoid any loss of marginal benefit while keeping the biopsy process in streamline. Based on our analysis, we conclude that BAL cytology do not have additional diagnostic benefit for malignancy when adopting a comprehensive bronchoscopy approach. Excluding infection is a more important application for BAL in patients with suspected thoracic malignancy, and it’s not safe to restrain BAL culture only in patients with pre-test suspicion for infection. We recommend lowering the threshold for BAL culture while integrating risk factors such as age, coexisting COPD and DM.
Acknowledgements
The authors thank Dr. Na You (Department of Biostatistics, Sun Yat-sen University, Guangzhou 86-510000, China) for assistance with statistical analysis.
Footnote
Conflicts of Interest: X Zhang received salary support from Program of National Key Clinical Specialties of China for this work. M Zeng and RC Yung received salary support from Philips NA institutional grant to Johns Hopkins University for the investigation of Imaging Enhanced Pulmonary Diagnostic Procedures. The other authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board at the Johns Hopkins Medical Institute approved this study (JHMI IRB NA_00026855). Consents were obtained from all patients.
References
- Pirozynski M. Bronchoalveolar lavage in the diagnosis of peripheral, primary lung cancer. Chest 1992;102:372-4. [Crossref] [PubMed]
- de Gracia J, Bravo C, Miravitlles M, et al. Diagnostic value of bronchoalveolar lavage in peripheral lung cancer. Am Rev Respir Dis 1993;147:649-52. [Crossref] [PubMed]
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S-128S. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Treat J, Scagliotti GV, Peng G, et al. Comparison of pemetrexed plus cisplatin with other first-line doublets in advanced non-small cell lung cancer (NSCLC): a combined analysis of three phase 3 trials. Lung Cancer 2012;76:222-7. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [Crossref] [PubMed]
- Rasmussen TR, Korsgaard J, Møller JK, et al. Quantitative culture of bronchoalveolar lavage fluid in community-acquired lower respiratory tract infections. Respir Med 2001;95:885-90. [Crossref] [PubMed]
- Baselski VS, Wunderink RG. Bronchoscopic diagnosis of pneumonia. Clin Microbiol Rev 1994;7:533-58. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68 Suppl 1:i1-i44. [Crossref] [PubMed]
- Clinical guidelines and indications for bronchoalveolar lavage (BAL): Report of the European Society of Pneumology Task Group on BAL. Eur Respir J 1990;3:937-76. [PubMed]
- Stolz D, Stulz A, Müller B, et al. BAL neutrophils, serum procalcitonin, and C-reactive protein to predict bacterial infection in the immunocompromised host. Chest 2007;132:504-14. [Crossref] [PubMed]
- British Thoracic Society Bronchoscopy Guidelines Committee. a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56 Suppl 1:i1-21. [Crossref] [PubMed]
- Poletti V, Poletti G, Murer B, et al. Bronchoalveolar lavage in malignancy. Semin Respir Crit Care Med 2007;28:534-45. [Crossref] [PubMed]
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. [Crossref] [PubMed]
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012;10:240-65. [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Boonsarngsuk V, Raweelert P, Juthakarn S. Endobronchial ultrasound plus fluoroscopy versus fluoroscopy-guided bronchoscopy: a comparison of diagnostic yields in peripheral pulmonary lesions. Lung 2012;190:233-7. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]
- Cornwell LD, Bakaeen FG, Lan CK, et al. Endobronchial ultrasonography-guided transbronchial needle aspiration biopsy for preoperative nodal staging of lung cancer in a veteran population. JAMA Surg 2013;148:1024-9. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Chee A, Stather DR, Maceachern P, et al. Diagnostic utility of peripheral endobronchial ultrasound with electromagnetic navigation bronchoscopy in peripheral lung nodules. Respirology 2013;18:784-9. [Crossref] [PubMed]
- Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013;188:327-33. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Feinstein MB, Mokhtari M, Ferreiro R, et al. Fiberoptic bronchoscopy in allogeneic bone marrow transplantation: findings in the era of serum cytomegalovirus antigen surveillance. Chest 2001;120:1094-100. [Crossref] [PubMed]
- Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712-22. [Crossref] [PubMed]
- Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med 2008;36:100-7. [Crossref] [PubMed]
- Hofmeister CC, Czerlanis C, Forsythe S, et al. Retrospective utility of bronchoscopy after hematopoietic stem cell transplant. Bone Marrow Transplant 2006;38:693-8. [Crossref] [PubMed]
- Poletti V, Romagna M, Allen KA, et al. Bronchoalveolar lavage in the diagnosis of disseminated lung tumors. Acta Cytol 1995;39:472-7. [PubMed]
- Tachihara M, Ishida T, Kanazawa K, et al. A virtual bronchoscopic navigation system under X-ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer 2007;57:322-7. [Crossref] [PubMed]
- Dooms CA, Verbeken EK, Becker HD, et al. Endobronchial ultrasonography in bronchoscopic occult pulmonary lesions. J Thorac Oncol 2007;2:121-4. [Crossref] [PubMed]
- Asano F, Matsuno Y, Shinagawa N, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006;130:559-66. [Crossref] [PubMed]
- Kikuchi E, Yamazaki K, Sukoh N, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J 2004;24:533-7. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Sagerup CM, Småstuen M, Johannesen TB, et al. Increasing age and carcinoma not otherwise specified: a 20-year population study of 40,118 lung cancer patients. J Thorac Oncol 2012;7:57-63. [Crossref] [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]
- Carpagnano GE, Lacedonia D, Palladino GP, et al. Aspergillus spp. colonization in exhaled breath condensate of lung cancer patients from Puglia Region of Italy. BMC Pulm Med 2014;14:22. [Crossref] [PubMed]
- Maskell NA, Waine DJ, Lindley A, et al. Asymptomatic carriage of Pneumocystis jiroveci in subjects undergoing bronchoscopy: a prospective study. Thorax 2003;58:594-7. [Crossref] [PubMed]
- Shahid M, Malik A, Bhargava R. Bronchogenic carcinoma and secondary aspergillosis--common yet unexplored: evaluation of the role of bronchoalveolar lavage-polymerase chain reaction and some nonvalidated serologic methods to establish early diagnosis. Cancer 2008;113:547-58. [Crossref] [PubMed]
- Malik A, Shahid M, Bhargava R. Prevalence of aspergillosis in bronchogenic carcinoma. Indian J Pathol Microbiol 2003;46:507-10. [PubMed]



