Impact of EGFR mutation status on tumor response and progression free survival after first-line chemotherapy in patients with advanced non-small-cell lung cancer: a meta-analysis
Introduction
Lung cancer, predominantly non-small-cell lung cancer (NSCLC), is the leading cause of cancer-related mortality worldwide (1). The majority of patients are diagnosed at advanced stages in which there are few treatment options (2). Despite the limited efficacy, platinum-based doublet chemotherapy remains the standard first-line treatment for advanced NSCLC in recent years (3,4). Advances in genetic testing allowed the discovery of existence and clinical significance of driver oncogenes which could be selected as a therapeutic target, such as activated epidermal growth factor receptor (EGFR) mutations (5). It has been extensively proved that NSCLC patients who harbor sensitive EGFR mutations (exon 19 deletion or L858R mutation in exon 21) derive greater benefits from EGFR-tyrosine kinase inhibitors (EGFR-TKIs), such as erlotinib and gefitinib, than those with wild type tumors (6,7). The predictive value of EGFR mutation status for EGFR-TKIs efficacy has been substantially confirmed.
In contrast, people used to believe there is no correlation between EGFR mutation status and cytotoxic chemotherapy. Data from some previous studies suggested that Asians represented higher response rate than Caucasians in receiving chemotherapy (8). From the present point of view, the most prominent intrinsic genetic variance between these two races is the proportion of patients with EGFR mutations. Considering the huge differences in tumor biology between EGFR mutation-positive and -negative NSCLC, it is interesting to investigate whether EGFR mutation status also influence chemotherapy efficacy. Several recent studies revealed that advanced NSCLC patients with positive EGFR mutation had favorable response to first-line cytotoxic chemotherapy compared with wild type patients (9,10), while another study showed contrary results (11). In addition, another clinical research reported that there was no obvious association between EGFR mutation status and first-line chemotherapy response in NSCLC (12). Therefore, whether EGFR mutation status is associated with responsiveness to front-line chemotherapy in advanced NSCLC is still not clear. A comprehensive analysis of the various outcomes is warranted. Thus, we sought to perform a meta-analysis incorporating all available evidences to evaluate the clinical outcome according to the EGFR mutation status in patients with advanced NSCLC treated with front-line conventional chemotherapy.
Methods
Literature search
All relevant articles were retrieved by searching PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library using a combination of the terms “EGFR”, “epidermal growth factor receptor”, “mutation”, “lung”, “non-small-cell lung cancer”, “NSCLC” and “chemotherapy”. An additional search through Google Scholar and a manual search through reference lists of relevant reviews and included studies were additionally performed. Two authors (ZY and KS) carried out the search independently. No restriction by language or year was set in the search.
Inclusion and exclusion criteria
Eligible studies should meet the following criteria: (I) studies which investigate or report a subset of patients with first-line chemotherapy without combination of EGFR inhibitors (e.g., TKIs or monoclonal antibodies) or other agents potentially targeting the EGFR pathway (e.g., multitargeted antiangiogenic TKIs) in patients with local advanced or metastatic (IIIB or IV) NSCLC; (II) prior neoadjuvant or adjuvant chemotherapy in patients with recurrence after surgery was permitted if it had elapsed from last administration to relapse at least 6 months; (III) EGFR mutation analysis was performed on available tumor tissue samples instead of circulating free DNA in serum in first-line chemotherapy treatment cohort; (IV) at least one primary outcomes was available. Studies failed to meet the inclusion criteria will be excluded.
Outcomes measures, data extraction and quality assessment
Primary outcomes for this meta-analysis were objective response rate (ORR), namely partial response (PR) plus complete response (CR), and 6-month progression-free survival (PFS) rate. The data collection and assessment of methodological quality followed the QUORUM and the Cochrane Collaboration guidelines (http://www.cochrane.de). The data on study type, treatment regimens, major clinical features, ORR and 6-month PFS rate were extracted by two investigators (FW and PH) independently. Figures were electronically digitized and Kaplan-Meier curves were downloaded by appropriate software (Engauge Digitizer, ver 2.12, Mark Mitchell, 2002, free software down loaded from http://sourceforge.net). Two reviewers (SW and DQ) used a JADAD score to evaluate the quality of randomized controlled trials (RCTs) and a modified Newcastle-Ottawa scale to assess the quality of non-RCT studies (13). Discrepancies were discussed by all investigators to reach consensus.
Statistical analysis
In consideration of any potential heterogeneity, we conducted this meta-analysis with a random-effect model in order to avoid any potential heterogeneity. The results were reported as pooled odds radios (ORs) with the corresponding 95% confidence interval (CI). Subgroup and sensitivity analysis were stratified for literature type, EGFR mutation analysis method, therapeutic regimen, patient origins. An OR greater than one reflected a better ORR or 6-month PFS rate in the EGFR mutant arm. Statistical heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). Statistical significance was considered at P<0.05. All calculations were performed using REVIEW MANAGER (version 5.0 for Windows; the Cochrane Collaboration, Oxford, UK).
Publication bias
An extensive search strategy was made to minimize the potential for publication bias. Graphical funnel plots were generated to visually assess a publication bias (14). The statistical methods to detect funnel plot asymmetry were the rank correlation test of Begg and Mazumdar and the regression asymmetry test of Egger (14,15).
Results
Eligible studies
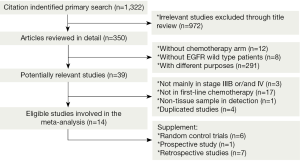
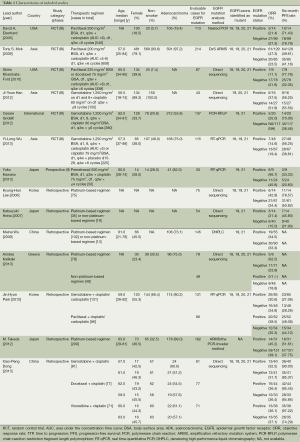
We identified 1,322 records according to the search strategy and finally included 14 studies (six RCTs, one prospective study and seven retrospective studies) involving 1,772 advanced NSCLC patients who had been tested for EGFR mutations in first-line chemotherapy treatment cohort (9-12,16-25). Figure 1 summarized the flow chart. Among these studies, chemotherapy regimens were platinum-based doublets at standard dose, namely cisplatin/carboplatin plus one of the third generation agents (including gemcitabine, paclitaxel, docetaxel, vinorelbine, and pemetrexed), or some non-platinum based regimens. Regimens were not specific in five retrospective studies (10,21-24) so that they were excluded in subgroup analysis stratified for therapeutic regimen. Detecting approaches for EGFR mutation included direct sequencing, nested polymerase chain reaction (PCR), amplification refractory mutation system (ARMS), polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP); real time-quantitative PCR (RT-qPCR), denaturing high-performance liquid chromatography (DHPLC), which were also a sub-grouping factor. We considered time to progression (TTP) as PFS in studies by Eberhard (11) and Lee (21). Table 1 summarized the characteristics of all involved studies.
Full table
Objective response rate and six-month PFS rate
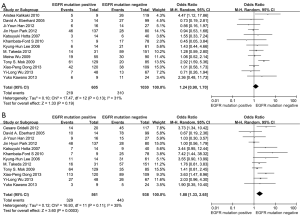
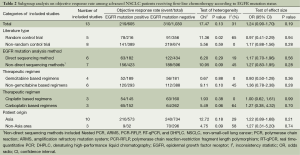
According to all literature with available data, patients with positive EGFR mutation had higher pooled ORR than wild type patients (35.8% vs. 30.1%), but there was no significant difference between the two groups (OR 1.24, 95% CI, 0.90 to 1.70; P=0.19; heterogeneity: Chi2 =17.47, P=0.13, I2 =31%; Figure 2A). Subgroup analyses stratified by study type (RCT vs. non-RCT), EGFR mutation detecting method (direct sequencing vs. non-sequencing methods), therapeutic regimen (gemcitabine-based vs. non-gemcitabine-based regimens and cisplatin-based vs. carboplatin-based regimens) and patient origin (Asians vs. non-Asians) consistently revealed no significant difference between the mutant group and wild type group (Table 2). EGFR mutants had higher 6-month PFS rate than wild type patients (62.1% vs. 45.1%) with significance (OR 1.88, 95% CI, 1.33-2.65; P=0.0003; heterogeneity: Chi2 =16.93, P=0.11, I2 =35%; Figure 2B). Subgroup analyses also revealed similar tendency of significantly superior 6-month PFS of EGFR mutants, regardless of study types, methods of EGFR mutation detection, chemotherapy regimens and patient origins (Table 3). Additionally, we pooled the results of DCR although only five studies reported this data. No differences between EGFR mutation positive and negative groups were observed (OR 1.33, 95% CI, 0.93-1.91; P=0.11; heterogeneity: Chi2 =2.23, P=0.69, I2 =0%; Figure 3).
Full table
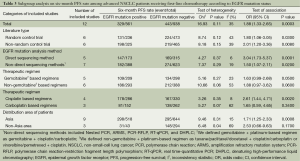
Full table
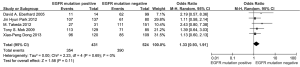
Assessment of heterogeneity and publication bias
As described above, the statistical heterogeneity was moderate. Any potential clinical heterogeneity was examined and subsequently excluded by subgroup analyses. In addition, sensitivity analysis by leaving any study out did not alter the general results. There was no publication bias for both outcome measures, with asymmetrical appearance on funnel plot analysis (Figure 4) and all P values greater than 0.05 in Begg’s test and Egger’s test.
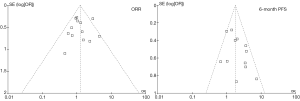
Discussion
The association of EGFR mutation status with the responsiveness or prognosis in patients with advanced NSCLC after first-line chemotherapy was controversial based on previous small-size reports. A meta-analysis that could incorporate all available results, including subgroup data from RCTs as well, is a good way to address our concerns. In the current study, we found that 6-month PFS rate was significantly higher in EGFR mutants than in wild type patients after first-line chemotherapy, while the ORR and DCR appeared to be higher but the difference did not reach significance. These results admit of two interpretations.
Firstly, EGFR mutation might indeed be a predictor to the efficacy of cytotoxic chemotherapy. Activation of EGFR-dependent pathway plays an important role in the proliferation and aggressive phenotype transition of epithelial cells especially EGFR-mutated tumors (26,27). Moreover, a prior research indicated that a critical level of EGFR signaling was necessary for cisplatin-mediated apoptosis in tumor cells and suggested an inhibitory effect of this pathway on the repair of cisplatin-damaged DNA (28). Therefore, it was reasonable to hypothesize that tumor cells harboring EGFR mutation are more sensitive to cytotoxic chemotherapy. The hypothesis for selective killing of EGFR+ cells was supported by a clinical observation which showed a reduced plasma EGFR mutation frequency after chemotherapy in patients with NSCLC (29). By selectively eliminating or suppressing the ‘seeds’, tumor growths were persistently restricted, which translated into prolonged PFS as our result indicated. On the other hand, EGFR mutants did have higher pooled response rate although the magnitude of benefit was not as great as that of PFS. We suspected that the magnitude difference was attributed to the intratumoral heterogeneity. A recent study demonstrated that approximately 30% of patients presented intratumoral EGFR mutational heterogeneity through microdissection of the tumor samples (30). Therefore, tumors detected as EGFR mutated not necessarily contain pure EGFR+ cells. In other words, the intratumoral abundace of EGFR+ cells might be small in some patients. Thus, selective killing of EGFR+ cells was probably not associated with significant tumor shrinkage. As a result, patients intrinsically ‘responded’ to the chemotherapy might fail to meet the criteria for ORR (at least a 30% decrease in the sum of diameters of target lesions) according to Recist 1.1 criteria (31). However, direct evidence to confirm this mechanism requires real-time re-biopsy after treatments, which seems to be an impossible mission considering ethics. Secondly, we can not rule out the possibility that the improved PFS was merely the underlying prognostic effect of EGFR mutation since there was evidence showing that EGFR mutation was likely to be a favorable prognostic factor (32). However, the prognostic value of EGFR mutation itself in NSCLC was still controversial (33).
Nonetheless, regardless of what the true causes are, this comprehensive analysis confirmed the association between EGFR mutation and PFS. This was highly concordant with an important report this year that among the patients treated with non-targeted therapy, those with a driver mutation detected had a longer median overall survival than those without identified driver mutations (2.4 vs. 2.1 years) (34). All these results gave us some important hints. Firstly, we strongly suggested that investigators should consider the proportion of EGFR mutation patients as a stratification factor in designing or reviewing clinical studies regarding chemotherapy regimen or other non-targeted agents. Second, it might partially explain why some clinical trials on chemotherapy in Asia reported higher response rate than those in Europe-American, and similarly, explain the negative results of combination of gefitinib with chemotherapy in patients with EGFR mutation compared with chemotherapy alone in some previous studies (35). In addition, the response to chemotherapy in EGFR wild type patients or projectively driven mutation ‘pan-negative’ patients was worse than what we acknowledged. Therefore, more efforts should be made to improve the prognosis of this population.
Notably, we only focused on first-line chemotherapy in this analysis in order to minimize the crossover effects. Some previous investigations suggested an inferior response from EGFR-TKIs following treatment of chemotherapy (36). Consistently, the study by Bai et al. also showed that the overall incidence of EGFR mutation was lower in plasma DNA after first-line chemotherapy (29). Thus, getting second-line or third-line chemotherapy involved will tangle the discussion.
This is the first study to comprehensively answer the impact of EGFR mutation on chemotherapy, addressing the confusion from inconsistent conclusions of current studies. However, there are several limitations. First, our meta-analysis was based on non-randomized studies and sub-group data extracted from RCTs, which somehow compromised the evidence level. Second, EGFR exons identified as mutant were heterogeneous among included articles but we were unable to assess whether 19 or 21 exon alterations had different impact on chemotherapy. Finally, we failed to investigate different first-line regimens separately with limited data. In addition, we cannot differentiate the respective impact of EGFR mutation on cell-cycle nonspecific antineoplastic agents (platinum) and specific agents (third-generation agents). For clinical practice, after all, it is essential to determine the optimal regimen for EGFR mutant NSCLC patients, especially who have failed front-line EGFR-TKIs or have no access to these agents. Further studies are warranted.
In conclusion, this meta-analysis showed that advanced NSCLC patient with EGFR mutation had significantly higher 6-month PFS rate and potentially higher ORR than wild type patients after first-line chemotherapy. We suggest that EGFR mutation status should be considered a stratification factor not only in studies regarding EGFR-targeted agents but also in those regarding non-EGFR-targeted drugs.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Wakelee H, Belani CP. Optimizing first-line treatment options for patients with advanced NSCLC. Oncologist 2005;10 Suppl 3:1-10. [PubMed]
- Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285-91. [PubMed]
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330-53. [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [PubMed]
- Paz-Ares L, Soulières D, Melezínek I, et al. Clinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med 2010;14:51-69. [PubMed]
- Soo RA, Loh M, Mok TS, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol 2011;6:1030-8. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Hotta K, Kiura K, Toyooka S, et al. Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small cell lung cancer. J Thorac Oncol 2007;2:632-7. [PubMed]
- Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900-9. [PubMed]
- Park JH, Lee SH, Keam B, et al. EGFR mutations as a predictive marker of cytotoxic chemotherapy. Lung Cancer 2012;77:433-7. [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.doc
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Khambata-Ford S, Harbison CT, Hart LL, et al. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:918-27. [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [PubMed]
- Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012;30:3002-11. [PubMed]
- Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86. [PubMed]
- Kawano Y, Ohyanagi F, Yanagitani N, et al. Pemetrexed and cisplatin for advanced non-squamous non-small cell lung cancer in Japanese patients: phase II study. Anticancer Res 2013;33:3327-33. [PubMed]
- Lee KH, Han SW, Hwang PG, et al. Epidermal growth factor receptor mutations and response to chemotherapy in patients with non-small-cell lung cancer. Jpn J Clin Oncol 2006;36:344-50. [PubMed]
- Wu M, Zhao J, Song SW, et al. EGFR mutations are associated with prognosis but not with the response to front-line chemotherapy in the Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2010;67:343-7. [PubMed]
- Kalikaki A, Koutsopoulos A, Hatzidaki D, et al. Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer 2010;69:110-5. [PubMed]
- Takeda M, Okamoto I, Sakai K, et al. Clinical outcome for EML4-ALK-positive patients with advanced non-small-cell lung cancer treated with first-line platinum-based chemotherapy. Ann Oncol 2012;23:2931-6. [PubMed]
- Dong X, Zhao X, Hao Y, et al. Response to first-line chemotherapy in patients with non-small-cell lung cancer according to epidermal growth factor receptor and K-RAS mutation status. Clin Lung Cancer 2013;14:680-7. [PubMed]
- Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 2006;9:485-95. [PubMed]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001;37 Suppl 4:S9-15. [PubMed]
- Dixit M, Yang JL, Poirier MC, et al. Abrogation of cisplatin-induced programmed cell death in human breast cancer cells by epidermal growth factor antisense RNA. J Natl Cancer Inst 1997;89:365-73. [PubMed]
- Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol 2012;30:3077-83. [PubMed]
- Bai H, Wang Z, Wang Y, et al. Detection and clinical significance of intratumoral EGFR mutational heterogeneity in Chinese patients with advanced non-small cell lung cancer. PLoS One 2013;8:e54170. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Izar B, Sequist L, Lee M, et al. The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg 2013;96:962-8. [PubMed]
- Selvaggi G, Novello S, Torri V, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol 2004;15:28-32. [PubMed]
- Johnson BE, Kris MG, Berry LD, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium (LCMC). J Clin Oncol 2013;31:490s.
- Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 2005;23:8081-92. [PubMed]
- Chin TM, Quinlan MP, Singh A, et al. Reduced Erlotinib sensitivity of epidermal growth factor receptor-mutant non-small cell lung cancer following cisplatin exposure: a cell culture model of second-line erlotinib treatment. Clin Cancer Res 2008;14:6867-76. [PubMed]