|
Original Article
Clinicopathologic analysis of cardiac myxomas: Seven years’ experience with 61 patients
Ji-Gang Wang1, Yu-Jun Li1, Hui Liu1, Ning-Ning Li2, Jie Zhao1, Xiao-Ming Xing1
1Department of Pathology, the Affiliated Hospital of Medical College, Qingdao University, 16 Jiangsu Road, Qingdao, Shandong Province, 266003, China; 2Department of Nephrology, the Affiliated Hospital of Medical College, Qingdao University, 16 Jiangsu Road, Qingdao, Shandong Province, 266003, China
Corresponding to: Ji-Gang Wang. Department of Pathology, the Affiliated Hospital of Medical College, Qingdao University, 16 Jiangsu Road, Qingdao, Shandong Province, 266003, China. Tel: +86-532-82911532; Fax: +86-532-82911533. Email: qdwangjigang@hotmail.com.
|
|
Abstract
Objective: Cardiac myxomas are the most common primary neoplasms of heart. The present study was performed on the 61 cases of patients with cardiac myxoma, in order to investigate the tumors’ clinical and pathological features, and to identify the relationship between the pathological characteristics and clinical behaviors.
Methods: A total of 61 cardiac myxoma cases were analyzed and reviewed retrospectively, including the clinical presentations, physical examinations, and echocardiography, electrocardiography, and pathology documents.
Results: The total patient cohort was made up of 37 women and 24 men. The average age at diagnosis was 48.8 years in males and 51.9 years in females. The most common complaint was dyspnea (37 cases, 60.7%) and the most common sign was systolic murmur (30 cases, 49.2%). Two surface structures and three tumor cell arrangement patterns were observed, and statistical analysis revealed the surface structure was related to the cell arrangement pattern. However, neither the cell arrangement pattern nor the tumor surface structure showed a significant correlation with the clinical presentation.
Conclusions: The present study showed the pathological profiles of cardiac myxomas were not related to the clinical presentations. The results of our study indicate morphologic classifications of cardiac myxomas may not be significant for clinical practice.
Key words
Cardiac neoplasms; myxoma; immunohistochemistry; pathology, surgical; neoplasm recurrence, local
J Thorac Dis 2012;4(3):272-283. DOI: 10.3978/j.issn.2072-1439.2012.05.07 |
|
Introduction
Primary tumors of the heart are extremely rare, with an estimated incidence ranged from 0.0017% to 0.33% at autopsy ( 1). Cardiac myxomas represent the most frequent benign cardiac tumors. In most surgical series, they account for almost 80% of cases ( 2). The cells giving rise to the tumor are considered to be multipotential mesenchymal cells that persist as embryonal residues during septation of the heart ( 3, 4). They also are thought to arise from cardiomyocyte progenitor cells, subendothelial vasoformative reserve cells or primitive cells which reside in the fossa ovalis and surrounding endocardium or endocardial sensory nerve ( 5- 8). Occasionally, mucous glandular epithelium may present, which may represent rests of entrapped embryonic foregut ( 9, 10). Two types of macroscopic appearance are observed: polypoid type and papillary type ( 11, 12). The histopathological diagnosis of a cardiac myxoma depends on the identification of the myxoma cell, which has occasionally been called the lepidic cell ( 13). The cells are arranged singly or in small clusters, or formed capillary like channels ( 2). Some morphological and immunohistochemical features may be related to the clinical presentations. Burke found that embolic myxomas were less often fibrotic than nonembolic myxomas and were more likely thrombosed and extensively myxoid with an irregular frond-like surface. Fibrotic and non-thrombosed tumors had a longer mean duration of clinical symptoms and were found in older persons. Recurrent, multiple, and familial myxomas were more often found in younger women and, more likely irregular surfaced and histologically myxoid ( 4). Endo’s group reported that tumors associated with constitutional signs were significantly more likely to be large, multiple, or recurrent than those unassociated with
constitutional signs ( 14). Papillary surface myxomas are thought
to be related to embolism, and large left atrial tumors are related
to atrial fibrillation. Myxoma cells usually express IL-6, and some
tumors have abnormal cellular DNA content ( 15). A C769T
PRKAR1a mutation has been observed in “familial myxomas” ( 16). Previous studies have reported a large series of myxomas,
however, little was focusing on the histopathologic classifications,
and the origin of the myxoma cells is still controversial ( 8).
Moreover, there is still no study related to cardiac myxoma in
Shandong Peninsula. Therefore, we presented a retrospective
review of our institution’s experience to investigate the clinical
and pathological features of cardiac myxomas, and to identify the
relationship between the pathological characteristics and clinical
presentations. |
|
Materials and methods
Materials
61 consecutive cases of cardiac myxomas, surgically resected
at the Cardiac Surgery Department of the Affiliated Hospital
of Medical College, Qingdao University, were identified by
searching the surgical pathology database during the seven-year
period between 2004 through 2010. During the seven years’
period, a total of 71 primary cardiac or pericardiac tumors had
been identified, including myxomas (61 cases, occupied about
85.9%), malignant fibrohistiocytomas (2 cases), non-Hodgkin’s
lymphomas (2 cases), low grade myxofibrosarcoma (1 case),
liposarcoma (1 case) ( 17), angiosarcoma (1 case), hemangioma
(1 case), papillary fibroelastoma (1 case), and rhabdomyoma (1
case). Approval for this study was obtained from the Institutional
Review Board. The medical records were reviewed with clinical
presentations, methods of diagnosis, management. Followup
was made by contacting with referring physicians or by
telephone. Pathologic study
All tumors underwent surgical resection after imaging diagnosis.
The tumor location and maximal diameter were evaluated in
all cases. All specimens were fixed in 10% buffered formalin
and embedded in paraffin, and then treated with Hematoxylin
and Eosin (H&E) staining and immunohistochemical staining.
The latest ten cases were treated with Alcian blue-Periodic acid
schiff (AB-PAS) staining to determine and identify carbohydrate
macromolecules of the myxoid stroma. When detected by ABPAS
staining, neutral mucopolysaccharides are stained “purplemagenta”
by schiff ’s reagent, acid mucopolysaccharides are
stained “blue” by alcian blue, and the complex of the both are
stained “purplish red”. A panel of antibodies, including CD34,
CD68, smooth muscle actin (SMA), epithelial membrane
antigen (EMA), Vimentin, and cytokeratin (CK), was used for
immunohistochemical staining (all antibodies were from Santa
Cruz, Beijing, China). The specificity of immunolabeling was
demonstrated by the absence of labeling of the antigen when the
primary antibody was omitted. All slides were reviewed by two
independent pathologists using an Olympus BX-51 microscope
(Tokyo, Japan). Only the brown particles that were easily visible
with a low power objective was categorized positive staining.
Statistical analysis
Data were analyzed using SPSS software for Microsoft Windows.
Mean values between two groups were compared by Student
t-tests. The associations between the morphological features and
clinical presentations were determined by Pearson’s chi-square
tests with correction for the continuity. The criterion level of
significance was P<0.05 for all comparisons. |
|
Results
Clinical findings
60 cases were sporadic myxomas and 1 case was familial
myxoma. The total patient cohort was made up of 37 women
and 24 men (female/male ratio was 1.5). Patients’ age ranged
from 12 to 76 years (mean age was 50.7±15.7 years). Most
patients were in the fifth, sixth and seventh decades of life (39 of
the total 61 cases, account for 60.7%). It was rare in individuals
younger than 30 years (5 cases, less than 10%). The average age
at diagnosis was 48.8 years in males and 51.9 years in females
(P=0.46, no significant difference between males and females).
The myxomas involved predominantly the left atrial cavities
(54 cases, 88.5%). Besides, there were six right atrial myxomas
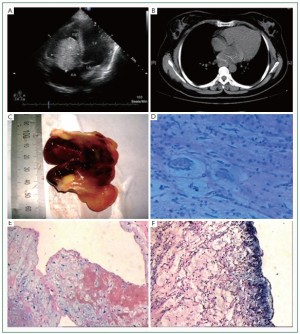
(9.8%) and one right ventricular myxoma (1.7%) ( Figure 1A).
The detailed descriptions of the six right atrial myxomas and one
right ventricular myxoma are offered in Table 1. Most myxomas
were attached to the septum by a stalk (57 cases, 93.4%). The
right ventricular myxoma was originated from the regulating
bundle branch.
| Table 1. Clinicopathologic features of 6 right atrial and 1 right ventricular myxomas. |
| Age (years)/sex |
Symptoms/ signs |
Location |
Tumor size (cm) |
Macro/histopathology |
Follow-up |
| 69/Male |
Dyspnea and palpitation |
Right atrium (atrial
septum) |
6×3×3 |
Papillary/significant
hemorrhage and
necrosis |
Recovered |
| 30/Male |
Found during
echocardiography after
a “biatial and right
ventricular myxoma
excision” operation seven
years ago |
Right atrium (atrial
septum), tricuspid valve |
4×4×2, 1 |
Solid/ cell cord
predominant |
Recovered |
| 49/Female |
Asymptomatic |
Right atrium (atrial
septum) |
3.5×2.4×2 |
Papillary/significant
hemorrhage and
necrosis |
Recovered |
| 50/Male |
Dyspnea and angina |
Right atrium (atrial
septum) |
3×2.2×2 |
Solid/cell cord
predominant |
Recovered |
| 35/Female |
Dyspnea and palpitation |
Right atrium (atrial
septum) |
6×5×3 |
Papillary/single cell
predominant |
Recovered |
| 19/Female |
Cough |
Right atrium (atrial
septum) |
7×7×5 |
Papillary/cell cord
predominant |
Recovered |
| 34/Male |
Syncope |
Right ventricle, originated
from the regulating
bundle branch |
7×5×3.5 |
Solid/single cell
predominant |
Recovered |
Clinical presentations are described in Table 2. Dyspnea was
the most frequent complaint at diagnosis (60.7%). The course
of disease at presentation ranged from two days to twenty years.
Four cases (6.6%) were asymptomatic. Of these four myxomas,
three were located in the left atrium and one located in the right
atrium. The four myxomas were incidentally found during other
preoperative examination or routine examination.
| Table 2. Clinical presentations of 61 patients. |
| Symptoms |
Course of disease |
Total (61) |
|
Left Atrial (54) |
|
Right Atrial (6) |
|
Right Ventricular (1) |
| Cases |
% |
Cases |
% |
Cases |
% |
Cases |
% |
| Cardiac |
|
|
|
|
|
|
|
|
|
| Dyspnea |
5 days - 20 years |
37 |
60.7 |
34 |
63.0 |
3 |
50 |
- |
- |
| Palpitation |
2 days - 20 years |
28 |
45.9 |
26 |
48.1 |
2 |
33.3 |
- |
- |
| Cough |
8 days - 6months |
4 |
6.6 |
3 |
5.6 |
1 |
16.7 |
- |
- |
| Angina |
1 week - 30 years |
3 |
4.9 |
2 |
3.7 |
1 |
16.7 |
- |
- |
| Edema of lower limbs |
1 year |
1 |
1.6 |
1 |
1.6 |
- |
- |
- |
- |
| Central nervous |
|
|
|
|
|
|
|
|
|
| Vertigo |
6 months - 6 years |
4 |
6.6 |
4 |
7.4 |
- |
- |
- |
- |
| Cerebral infarction |
10 days - 7 months |
2 |
3.3 |
2 |
3.7 |
- |
- |
- |
- |
| Syncope |
3 days |
1 |
1.6 |
- |
|
- |
- |
1 |
100 |
| Systemic |
|
|
|
|
|
|
|
|
|
| Fatigue |
6 months - 2 years |
4 |
6.6 |
4 |
7.3 |
- |
- |
- |
- |
| Fever |
2 months - 4 months |
3 |
4.9 |
3 |
5.6 |
- |
- |
- |
- |
| Asymptomatic |
- |
4 |
6.6 |
2 |
3.7 |
2 |
33.3 |
- |
- |
Abnormal physical examination findings were recorded in 54
cases (88.5%). The most common sign was the systolic murmur
(30 cases, 49.2%), and then followed by the diastolic murmur
(19 cases, 31.1%). The murmur was often altered along with the body position. Tumor plop could be heard in 10 patients
(16.4%). Other abnormal cardiac signs included the loud first
sound (15 cases, 24.6%), the loud second sound (10 cases,
16.4%), and the opening snap (6 cases, 7.4%). Two-dimensional
echocardiography was performed in all patients, which could
well determine the tumor location, size, shape, attachment and
mobility. There was no misdiagnosis with echocardiography.
Radiological examinations (computed tomography or magnetic
resonance imaging) of the chest were only available in several
cases ( Figure 1B). Electrocardiography documents were
available in 55 cases, and abnormal findings were reported in 30
cases (54.5%), including signs of atrial hypertrophy (20 cases,
36.4%), atrial fibrillation (7 cases, 12.7%), arrhythmia (2 cases,
3.6%), and tachycardia (1 cases, 1.8%).
All patients underwent complete myxoma excisions with
cardiopulmonary bypass after echocardiographic diagnosis.
The patient with the recurrent right atrial myxoma also received
tricuspid valve repairing surgery. There were 50 cases available
for follow-up (ranged from 6 months to 7 years). Two cases died
of other diseases. There was one case bear a recurrence during
the follow-up period (2 years after the first surgery). The patient
underwent second surgery and recovered uneventfully after that.
Other 47 cases recovered uneventfully. Among them two cases
had accepted “myxoma resection” surgery at other institutions
7 years and 20 years before. Unfortunately, it is impossible for us
to get the detailed information at that time.
Gross pathology
The mean maximal diameter was 5.8±1.8 cm (ranged from 2 to
11 cm). There was no significant difference between the left and
right (left: 5.9±1.8 cm, right: 4.8±1.8 cm, P=0.13). The maximal
diameter of four asymptomatic cases was 6, 4, 3, and 3 cm,
respectively. On gross examination, the tumors had broad base,
and most have pedicles (57 cases). The tumor mass was soft,
gelatinous, and very friable. They were smooth and glistening
(solid type, 18 of 30 available cases with gross description) or
had multiple papillary, villous, finger-like projections (papillary
type, 12 of 30 cases). Two cases presented with cerebral
infarction symptoms were papillary type. The cut surface
was variable; most cases were soft, gelatinous, pale grey with
hemorrhage areas, others were a little firmer, or with calcification
( Figure 1B, 1C). Histopathology
On microscopy, myxoma was made up of a myxoid stroma
with variable myxoma cells. The stromal matrix was loose,
homogenized and red-stained, which contained variable
amounts of proteoglycans, collagen and elastin. Using ABPAS
staining, we found that the stromal matrix appeared to
be purplish red (positive for AB and PAS), while the mucous
halo showed blue color (positive for AB) ( Figure 1D). Variable
amounts of thin walled blood sinus or thick walled blood
vessels were often present. Hemorrhagic foci, fibrinoid necrosis,
hemasiderin-laden macrophages, and inflammatory cells were
also frequent. The tumor cells may be spindle, polygonal, or
stellate, and have round to oval nuclei with inconspicuous
nucleoli, eosinophilic cytoplasm, and indistinct borders. Mitoses
are rare. The cells could be arranged in single, in nests or cords,
or in vasoformative ring structures. Small or large mucous halos
were frequently observed to surround the myxoid cells, the cords
or vasoformative ring structures. In fact, most myxomas were
combined with the above two or three structures. 2 cases had
extensive hemorrhage and necrosis, which made it difficult to
classify. According to the arrangement pattern of tumor cells, we
categorized the other 59 myxomas into three subtypes: Single
cell predominant subtype, cell cord predominant subtype,
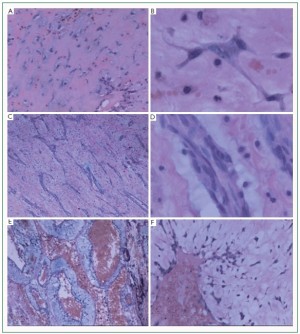
and vasoformative ring predominant subtype ( Figure 2). The
majority of myxomas were belonging to single cell predominant
subtype (28 cases, 47.5%). The cell cord predominant type often
showed rudimentary “chicken-wire” vessels like appearance, with
few and scattered single myxoid cells in the stroma. There were
21 cases belonging to this subtype (35.6%). The vasoformative
ring predominant subtype showed a hemangioma-like
appearance, containing distended blood sinus and positive alcian
blue stained myxiod halo. 10 cases were categorized into this
subtype (16.9%). Calcification was observed in 3 cases. Besides,
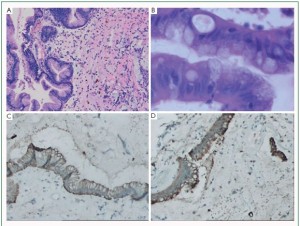
well-defined mucous glands, containing goblet cells interspersed
between cells, formed by columnar epithelium were found in 2
cases ( Figure 3A, 3B). According to the microscopic appearance of tumor surface, all
myxomas also could be divided into two subtypes: solid subtype
(29 cases) and papillary subtype (30 cases) ( Figure 1E, 1F).
Sometimes the tumor with clefted area made the differentiation
between the two subtypes difficult. In the present study, we
categorized such tumors into the former subtype. Only those
observed with obvious papillary architecture under low power
were categorized into papillary subtype. We found the two
subtypes have different cell arrangement patterns. The papillary
subtype appeared more likely to form a single cell predominant
pattern, whereas the solid subtype was more likely to form
rudimentary vessels ( Table 3). Moreover, statistical analysis
revealed there was no significant difference between these
morphological features and clinical symptoms ( Table 4).
| Table 3. Morphologic features of 59 myxomas (cases). |
| |
Solid |
Papillary |
| Single cell predominant |
7 |
21 |
| Cell cord predominant |
13 |
8 |
| Vasoformative ring predominant |
9 |
1 |
| The cell has expected count less than 5. Chi-square test showed the solid subtype and papillary subtype have different cell
arrangement architectures (x2=13.163, P=0.001). |
| Table 4. Correlation of clinical presentations and morphologic features (cases). |
| Symptoms |
Total |
Surface |
|
Cell arrangement |
P value |
| Solid (29) |
Papillary (30) |
Single (28) |
Cord (21) |
Ring (10) |
| Cardiac |
|
|
|
0.904 |
|
|
|
0.520 |
| Dyspnea |
37* |
18 (62.1%) |
18 (60.0%) |
19 (67.9%) |
11 (52.4%) |
6 (60.0%) |
| Palpitation |
28* |
13 (44.8%) |
14 (46.7%) |
14 (50.0%) |
9 (42.9%) |
4 (40.0%) |
| Cough |
4 |
3 (10.3%) |
1 (3.3%) |
3 (10.7%) |
1 (4.8%) |
0 |
| Angina |
3 |
2 (6.9%) |
1 (3.3%) |
0 |
3 (14.3%) |
0 |
| Edema of lower limbs |
1 |
1 (3.4%) |
0 |
0 |
0 |
3 (30.0%) |
| Central nervous |
|
|
|
|
|
|
| Vertigo |
4 |
2 (6.9%) |
2 (6.7%) |
1 (3.6%) |
1 (4.8%) |
2 (20.0%) |
| Cerebral infarction |
2 |
0 |
2 (6.7%) |
2 (7.1%) |
0 |
0 |
| Syncope |
1 |
1 (3.4%) |
0 |
1 (3.6%) |
0 |
0 |
| Systemic |
|
|
|
|
|
|
| Fatigue |
4 |
3 (10.3%) |
1 (3.3%) |
1 (3.6%) |
1 (4.8%) |
2 (20.0%) |
| Fever |
3 |
1 (3.4%) |
2 (6.7%) |
3 (10.7%) |
0 |
0 |
| Asymptomatic |
4* |
2 (6.9%) |
1 (3.3%) |
0 |
2 (9.5%) |
1 (10.0%) |
| *Case with extensive hemorrhage and necrosis was excluded. Little sample size made the exact analysis testing of every number difficult.
To reduce the errors, we combined data of each group, and performed Pearson’s chi-square tests with correction for the continuity. |
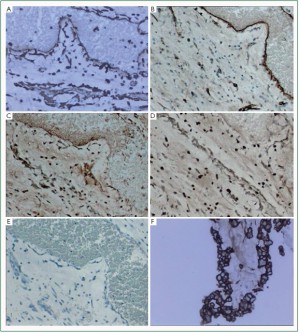
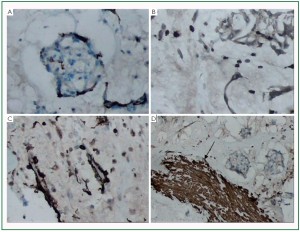
Immuohistochemical staining was carried out in 10 cases.
Vimentin was diffusely and strongly reactive with all tumor cells
and blood vessels, also reactive with surface lining cells. SMA,
CD34 and CD68 were expressed variably ( Figure 4, 5). Only
part of tumor cells showed positive immunoreactivity for these
antibodies. The surface lining cells were only positively stained
for Vimentin and CD34 ( Figure 4F). The glandular cells showed positive CK and EMA expressions ( Figure 3C, 3D). Recurrent myxomas
In our series, a 29-year-old male, who underwent a “biatrial and
right ventricular myxoma excision” operation at other institution
seven years ago (detailed operation and pathological information
at that time was hardly available), presented with a recurrent
tumor. He accepted a second surgery at our institution, and two
recurrent tumor masses which respectively located in the right
atrium and tricuspid valve were observed. Pathological analysis
showed the tumor masses have solid surface and rumentary
vessels, with foci of hemorrhage, hemorsiderin and patched
calcification, which were similar to other sporadic myxomas.
However, no pedicle was found. Although the patient denied
any family history, we still regard him as a familial myxoma case
according to the clinical features. A 16-year-old boy also bore
recurrence at the same place (the upper atrial septum) with a
pedicle (2 cm × 1.5 cm) after left myxoma resection two years
before. The patient presented in April 2006 with dyspnea and
palpitation over six months, and the tumor showed papillary
surface and single cell predominant structure. In May 2008 the
patient accepted the second surgery, however, the recurrent
tumor showed a solid appearance and cell cord predominant
arrangement structure. After that he recovered uneventfully.
We consider the recurrence may attribute to the residual tumor
cells. The other recurrent case was a 67-year-old female, who
present with dyspnea and palpitation, with a history of “left
atrial myxoma resection” twenty years before. The pathologic
examination revealed the tumor had a pedicle and appeared a
solid surface type and vasoformative ring predominant pattern.
Unfortunately, the previous excision record was not available.
The patient also recovered uneventfully after the second surgery.
Histologically, we found the recurrent cases had no malignant
histological characteristics, for instance, atypical nucleus and
pathologic karyokinesis.
|
|
Discussion
In the present study we have retrospectively reviewed a series
of 61 consecutive cardiac myxomas at a single institution of
Shandong Peninsula. To the best of our knowledge, this is one
of the largest populations related to myxomas during a decade’s
period. Our report is similar to the previous studies, which have
shown the tumors are the most common primary tumor, and
roughly 90% of the tumors are located in the atria, with the left
atrium accounting for 80% of those ( 18). Only 3-4% of myxomas
are detected in the left ventricle, and only 3-4% in the right ( 11).
Multilocular myxomas are extremely rare, which often result
in local recurrences ( 19). Familial myxomas are estimated to
account for 7% of atrial myxomas ( 20), are more often multiple,
recurrent and right sided, as compared to sporadic myxomas. The
affected patients are also younger, most presenting at 20-30 years
of age ( 21- 23). Moreover, our study demonstrated, for the first
time, that the surface appearance is associated with the tumor
cell arrangement structure. In addition, our study indicated that
the clinical presentations have no relations with the pathological
features, which may be different from the previous study ( 15).
The clinical presentation of myxomas is diverse and
dependent upon tumor location, size and mobility ( 24- 27).
According to a previous study, the most common symptom is
dyspnea (54%), and then followed by palpitation (35%) ( 15). Dyspnea and edema of lower limbs are thought to a consequence
of atrioventricular valve obstruction. Nevertheless, the intracardiac obstruction may also lead to narrowing outflow
tract and atrial fibrillation, which could contribute to dyspnea
and palpitation. Cough is thought to result in pulmonary venous
hypertension and frank pulmonary edema. Angina may be
caused by insufficient blood supply. Embolism is also a classic
symptom of myxomas, which have been reported to associate
with the papillary surface ( 15). The cause of some constitutional
disturbances is still unclear. Some findings suggest the cytokine
interleukin-6 (IL-6) may be responsible for that. The relationship
between IL-6 and constitutional syndromes is still controversial.
In some cases, the right atrial myxomas may induce pulmonary
hypertension because of embolism of tumor fragments. Right
ventricular myxomas may mimic stenosis of pulmonary valve
and cause syncope. In our series, both left and right atrial
myxomas were observed with symptoms of dyspnea, palpitation,
cough, and angina. Among them, one left atrial myxoma case
was observed with edema of lower limbs and syncope, however,
the patient also suffered from systemic lupus erythematosus
(SLE), which made the pathophysiological process even
puzzled. The symptoms disappeared after the myxoma resection.
Unfortunately, the patient died of SLE four years later. Besides,
two cases presented with cerebral infarction symptoms and one
case with angina and vertigo showed papillary surface, which was
consistent with previous studies ( 4, 15). However, in the present
study it is difficult to perform further exact analysis because of
the little sample size. Other cerebral symptoms in cases of solid
surface may only caused by cardiac obstruction and cerebral
ischemia. About 20% of cardiac myxomas are asymptomatic; they are
usually smaller than 4 cm ( 28, 29). The maximal diameter of four
asymptomatic cases of the present report was 6, 4, 3, and 3 cm,
respectively. We consider it is due to the small tumor size or long growth course, which may result in adaption to the tumor. In our study, 54.5% of patients exhibited abnormal
electrocardiography findings, compared with previous
study report of 56% ( 15). The abnormalities found on the
electrocardiography are usually nonspecific ( 11, 12, 15). They may
only reflect the hemodynamic alterations, such as atrial overload
or ventricular hypertrophy, which secondarily increased the
chamber diameter and altered conduction. Echocardiography is
widely recognized as a sensitive preoperative diagnostic method,
although it appears nonspecific to some occupying lesions. We
have an experience with a low grade cardiac myxofibrosarcoma,
which was considered as a myxoma on echocardiography.
Moreover, thrombus may be misdiagnosed as myxoma in some
cases. Nevertheless, echocardiography is also demonstrated as
the most accurate and reliable preoperative method with which
to predict the diameter, location, attachment, mobility, and
morphology of cardiac myxomas. Two surface types and three cell arrangement patterns
were observed in these cases. Statistical analysis revealed the
tumor cells of solid type have a tendency to form vasoforming
structures: the tumor cells may arranged in cords or rings.
However, the morphological features have no correlation
with the clinical presentations. The results indicate that the
morphologic classifications of cardiac myxomas may not be
significant for clinical practice. Imunohistochemical study
showed the tumor cells bear a diffuse, and positive expression
for Vimentin, and focal expressions for CD34, CD68, and
SMA. The results support the hypothesis of myxoma originated
from multipotential mesenchymal cells, capable of vessel,
smooth muscle, and histiocyte differentiation. The typical
mucous glands may represent rests of entrapped embryonic
foregut. Furthermore, previous reports have showed the cells
immunoreactive for neuropeptides (s-100, NSE) ( 7) and
Calrectinin ( 6, 30). Bone and brain metastases from glandular cardiac myxomas
have been reported in the recent literature ( 31- 35). The most
frequent metastatic site for cardiac myxomas is cerebrum ( 33).
Several reports have reviewed cerebral metastasis cases ( 34, 35).
Since most myxomas are located in the left atrium, systemic
embolism is particularly frequent. The tumor fragments
metastasized to cerebral vessel walls may penetrate through the
vessel wall, forming intra-atrial metastases. Some cytokines, such
as CXC chemokines, may explain the metastasis potential of
morphologically benign myxomas. In our series, no metastatic
case was observed, including two cases with typical mucous
glandular differentiation. Especially to deserve to be mentioned,
there was an uncommon histological likeness between the
glandular myxomas and mucinous adenocarcinomas. One case
with glandular differentiation in the series was considered as
metastatic mucinous adenocarcinoma at one time. In conclusion, we have retrospectively reviewed a series of
61 myxoma cases at a single institution of Shandong Peninsula.
The clinical presentations and pathological characteristics
(particular on the relationship between the both) were
investigated. Myxomas may cause varies kinds of symptoms,
and mainly categorize into two surface subtypes and three
cell arrangement patterns. However, further statistical analysis
showed these morphological features have no relationship with
the clinical presentations. The results of this study indicate the
histopathologic classifications of cardiac myxomas may not be
significant for clinical practice. |
|
Acknowledgements
We thank Professor Xiang-Rui Ji for his skillful assistance with
the English language.
Disclosure: We confirm that we have not previously published
or have not submitted the same manuscript elsewhere; we took
a significant part in the work and approved the final version of
the manuscript; we have complied with ethical standards; we
agree Pioneer Bioscience Publishing Company, to get a license
to publish the accepted article when the manuscript is accepted,
and we have obtained all necessary permissions to publish any
figures or tables in the manuscript, and assure that the authors
will pay for any necessary charges.
|
|
References
- Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol
1980;101:219-40.
- Burke AP, Tazelaar H, Gomez-Roman JJ, et al. Cardiac myxoma. In: Travis
WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology
and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon:
IARC Press, 2004: 260-3.
- Lie JT. The identity and histogenesis of cardiac myxomas: a controversy put
to rest. Arch Pathol Lab Med 1989;113:724-6.
- Burke AP, Virmani R. Cardiac myxoma. A clinicopathologic study. Am J
Clin Pathol 1993;100:671-80.
- Kodama H, Hirotani T, Suzuki Y, et al. Cardiomyogenic differentiation
in cardiac myxoma expressing lineage-specific transcription factors. Am J
Pathol 2002;161:381-9.
- Terracciano LM, Mhawech P, Suess K, et al. Calretinin as a marker for
cardiac myxoma. Diagnostic and histogenetic considerations. Am J Clin
Pathol 2000;114:754-9.
- Pucci A, Gagliardotto P, Zanini C, et al. Histopathologic and clinical
characterization of cardiac myxoma: review of 53 cases from a single
institution. Am Heart J 2000;140:134-8.
- Amano J, Kono T, Wada Y, et al. Cardiac myxoma: its origin and tumor
characteristics. Ann Thorac Cardiovasc Surg 2003;9:215-21.
- Johansson L. Histogenesis of cardiac myxomas. An immunohistochemical
study of 19 cases, including one with glandular structures, and review of the
literature. Arch Pathol Lab Med 1989;113:735-41.
- Pucci A, Bartoloni G, Tessitore E, et al. Cytokeratin profile and
neuroendocrine cells in the glandular component of cardiac myxoma.
Virchows Arch 2003;443:618-24.
- Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610-7.
- Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and
management. Lancet Oncol 2005;6:219-28.
- St John Sutton MG, Mercier LA, Giuliani ER, et al. Atrial myxomas: a
review of clinical experience in 40 patients. Mayo Clin Proc 1980;55:371-6.
- Endo A, Ohtahara A, Kinugawa T, et al. Characteristics of cardiac myxoma
with constitutional signs: a multicenter study in Japan. Clin Cardiol
2002;25:367-70.
- Acebo E, Val-Bernal JF, Gómez-Román JJ, et al. Clinicopathologic study
and DNA analysis of 37 cardiac myxomas: a 28-year experience. Chest
2003;123:1379-85.
- Yokomuro H, Yoshihara K, Watanabe Y, et al. The variations in the
immunologic features and interleukin-6 levels for the surgical treatment of
cardiac myxomas. Surg Today 2007;37:750-3.
- Wang JG, Wei ZM, Liu H, et al. Primary pleomorphic liposarcoma of
pericardium. Interact Cardiovasc Thorac Surg 2010;11:325-7.
- Kumar BV, Abbas AK, Fausto N, et al. Cardiac tumors. In: Kumar BV,
Abbas AK, Fausto N, Mitchell R, editors. Robbins basic pathology, 8th
edition. Philadelphia: Saunders, 2007: 417-8.
- Martin LW, Wasserman AG, Goldstein H, et al. Multiple cardiac myxomas
with multiple recurrences: unusual presentation of a “benign” tumor. Ann
Thorac Surg 1987;44:77-8.
- Casey M, Mah C, Merliss AD, et al. Identification of a novel genetic
locus for familial cardiac myxomas and Carney complex. Circulation
1998;98:2560-6.
- Larsson S, Lepore V, Kennergren C. Atrial myxomas: results of 25 years’
experience and review of the literature. Surgery 1989;105:695-8.
- Edwards A, Bermudez C, Piwonka G, et al. Carney’s syndrome: complex
myxomas. Report of four cases and review of the literature. Cardiovasc Surg
2002;10:264-75.
- Papagiannopoulos K, Hughes S, Nicholson AG, et al. Cystic lung lesions
in the pediatric and adult population: surgical experience at the Brompton
Hospital. Ann Thorac Surg 2002;73:1594-8.
- Premaratne S, Hasaniya NW, Arakaki HY, et al. Atrial myxomas:
experiences with 35 patients in Hawaii. Am J Surg 1995;169:600-3.
- Bjessmo S, Ivert T. Cardiac myxoma: 40 years’ experience in 63 patients.
Ann Thorac Surg 1997;63:697-700.
- Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac
myxoma. A series of 112 consecutive cases. Medicine (Baltimore)
2001;80:159-72.
- Gabe ED, Rodríguez Correa C, Vigliano C, et al. [Cardiac myxoma.
Clinical-pathological correlation]. Rev Esp Cardiol 2002;55:505-13.
- Goswami KC, Shrivastava S, Bahl VK, et al. Cardiac myxomas: clinical and
echocardiographic profile. Int J Cardiol 1998;63:251-9.
- Grebenc ML, Rosado-de-Christenson ML, Green CE, et al. Cardiac
myxoma: imaging features in 83 patients. Radiographics 2002;22:673-89.
- Acebo E, Val-Bernal JF, Gómez-Roman JJ. Thrombomodulin, calretinin
and c-kit (CD117) expression in cardiac myxoma. Histol Histopathol
2001;16:1031-6.
- Moiyadi AV, Moiyadi AA, Sampath S, et al. Intracranial metastasis
from a glandular variant of atrial myxoma. Acta Neurochir (Wien)
2007;149:1157–62.
- Uppin SG, Jambhekar N, Puri A, et al. Bone metastasis of glandular cardiac
myxoma mimicking a metastatic carcinoma. Skeletal Radiol 2011;40:107-11.
- Shimono T, Makino S, Kanamori Y, et al. Left atrial myxomas. Using
gross anatomic tumor types to determine clinical features and coronary
angiographic findings. Chest 1995;107:674-9.
- Altundag MB, Ertas G, Ucer AR, et al. Brain metastasis of cardiac myxoma:
case report and review of the literature. J Neurooncol 2005;75:181-4.
- Rodrigues D, Matthews N, Scoones D, et al. Recurrent cerebral metastasis
from a cardiac myxoma: case report and review of literature. Br J Neurosurg
2006;20:318-20.
Cite this article as: Wang JG, Li YJ, Liu H, Li NN, Zhao J, Xing XM.
Clinicopathologic analysis of cardiac myxomas: Seven years’ experience
with 61 patients. J Thorac Dis 2012;4(3):272-283. doi: 10.3978/
j.issn.2072-1439.2012.05.07
|