
The management of type 2 inflammatory respiratory diseases: a Chinese expert consensus [2024]
Highlight box
Key recommendations
• Type 2 (T2) inflammatory respiratory diseases are a set of inflammatory disorders in which the T2 immune response is the main pathogenesis. In the management of T2 inflammatory respiratory diseases, the characteristics of each type of T2 inflammatory respiratory disease and its comorbidities should be considered to select precise treatment, especially biological therapies targeting T2 inflammation.
What was recommended and what is new?
• Chronic respiratory diseases usually include a series of airway diseases such as asthma, chronic obstructive pulmonary disease and others. These diseases are heterogeneous and characterize by chronic airway and lung inflammations involving the infiltrations of multiple inflammatory cells and mediators. Pharmacological treatment for chronic respiratory diseases are mainly aimed at controlling airway inflammation (such as inhaled corticosteroids, and leukotriene receptor antagonists), broncho-dilating (such as long-acting beta-2-agonists and long-acting muscarinic antagonists) and relieving acute exacerbations (such as short-acting beta-agonists and systemic glucocorticoids).
• T2 inflammatory respiratory diseases are characterized by T2 inflammation. T2 inflammatory respiratory diseases include asthma, a subgroup of chronic obstructive pulmonary disease, allergic bronchopulmonary aspergillosis, eosinophilic granulomatosis with polyangiitis, eosinophilic bronchitis, eosinophilic pneumonia, and a subgroup of non-cystic fibrosis bronchiectasis. Therapies targeting T2 inflammation have demonstrated high efficacy in the treatment for T2 inflammatory respiratory diseases and their comorbidities.
What is the implication, and what should change now?
• T2 inflammation-targeted therapies show high potent in treatment for T2 inflammatory respiratory diseases and their comorbidities. However, there is still a lack of high-quality studies to evaluate the clinical effects. Moreover, biologics that can effectively treat all T2 inflammatory disorders need to be explored.
Introduction
Type 2 (T2) inflammatory respiratory diseases constitute a spectrum of inflammatory disorders that affect the airways and lung tissues, mediated by T2 inflammation, which refers to a specialized immune response involving the innate and the adaptive arms of the immune system to promote barrier immunity on mucosal surfaces (1). This category encompasses asthma, allergic bronchopulmonary aspergillosis (ABPA), eosinophilic granulomatosis with polyangiitis (EGPA), a subset of chronic obstructive pulmonary disease (COPD) and non-cystic fibrosis bronchiectasis, and other related conditions. Asthma and COPD exhibit a substantial global prevalence, while the diagnosis and treatment of ABPA and EGPA pose substantial clinical challenges. Hence, precise interventions by evolving understanding of their pathogenesis are imperative.
These T2 inflammatory respiratory diseases collectively affect nearly 1 billion individuals worldwide (2-5). Globally, the prevalence of asthma is 4.3% (2), and in China, it is 4.2% among individuals aged 20 and above, affecting over 45 million people (6). In 2015, the Global Burden of Disease (GBD) Study indicated that approximately 400,000 deaths occurred annually due to asthma worldwide, with in China, around 25,000 deaths were reported in 2019 (7). The annual death toll of COPD worldwide is 3.2 million (7), with 1.037 million deaths annually among COPD patients in China, accounting for approximately one-third of the global total (8). Although the mortality of asthma and COPD in China shows a downward trend year by year, the number of deaths shows an upward trend with age, and the situation was still serious (8). Moreover, misdiagnosis and missed diagnoses are widespread problems, which increase the concerns about the consequence of these diseases. A study conducted in China revealed that approximately 2.5% of asthma patients seeking medical treatment was diagnosed with ABPA (9). Although EGPA is not very common, it can affect the respiratory system and multiple tissues and organs in the body, resulting in a high consumption of medical resources. Studies have shown that between 17% to 42% of EGPA patients require frequent hospitalizations, while 25% to 42% need regular visits to the emergency department (5).
T2 inflammatory respiratory diseases frequently co-occur with other systemic conditions. Asthma, for instance, is frequently accompanied by lower respiratory tract disorders such as ABPA and COPD, allergic rhinitis (AR), chronic rhinosinusitis with nasal polyps (CRSwNP), and various digestive and skin disorders. These comorbidities significantly diminish patients’ quality of life, worsen disease severity and impose a greater medical burden than in patients without these co-morbidities (10).
In recent years, there has been a considerable increase in research focusing on disease mechanisms, biomarkers, and biological therapy, both domestically and internationally, promoting a deeper understanding of T2 inflammatory diseases, particularly relating to allergic and eosinophilic asthma. However, clinicians often need more awareness of the clinical significance of these advances, and there is still a gap in understanding the practical application of biologics targeting various T2 pathways. To assist clinical medical professionals in assessing and selecting biological therapies for such diseases, the Respiratory Allergy Disease Group (in preparation) of the Chinese Allergy Society convened 15 experts in respirology or allergy to discuss and draft a consensus report. This consensus offers comprehensive and systematic guidance to clinicians in understanding the immune response, pathological traits, and clinical manifestations of T2 inflammatory respiratory diseases. Additionally, it provides guidance and rational treatment strategies for selecting biological therapies for severe and refractory diseases based on standardized medical therapy. This consensus will adhere to continuous updates and evidence-based disease management principles, ensuring continuous supplementation and improvement in the future.
Methods
During the development of this consensus, a literature review group was constituted to formulate search strategies focusing on relevant issues related to T2 inflammatory respiratory diseases. PubMed was the leading platform to source relevant literature published within the past decade, commencing with the original literature referenced in domestic and foreign guidelines as supporting evidence. Subsequently, the search and screening process was expanded to cover a broader range of literature. Search items included “((Type 2 [Title/Abstract]) OR (Th2 [Title/Abstract]) OR (ILC2 [Title/Abstract])) AND ((asthma[Title/Abstract]) OR (chronic sinusitis with nasal polyps[Title/Abstract]) OR (CRSwNP[Title/Abstract]) OR (chronic sinusitis without nasal polyps[Title/Abstract]) OR (CRSwNP[Title/Abstract]) OR (allergic rhinitis[Title/Abstract]) OR (AR[Title/Abstract]) OR (allergic bronchopulmonary aspergillosis[Title/Abstract]) OR (ABPA[Title/Abstract]) OR (chronic obstructive pulmonary disease[Title/Abstract]) OR (COPD[Title/Abstract]) OR (Eosinophilic granulomatosis with polyangiitis[Title/Abstract]) OR (EGPA[Title/Abstract]) OR (Eosinophilic Bronchitis[Title/Abstract]) OR (EB[Title/Abstract]) OR (eosinophilic pneumonia[Title/Abstract]) OR (EP[Title/Abstract]) OR (non-cystic fibrosis bronchiectasis[Title/Abstract])) AND ((Omalizumab[Title/Abstract]) OR (Dupilumab[Title/Abstract]) OR (Mepolizumab[Title/Abstract]) OR (Benralizumab[Title/Abstract]) OR (Reslizumab[Title/Abstract]) OR (Tezepelumab[Title/Abstract]))”. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system provided a framework for assessing the quality of evidence and grading recommendations (11,12). Consensus was achieved through the expert consensus meeting approach. The consensus working group held multiple meetings to extensively discuss the critical issues of T2 inflammatory respiratory diseases. Two rounds of anonymous voting were carried out to facilitate the decision-making process. In the first round, focus issues regarding immune response mechanisms, pathophysiology, and clinical aspects were screened. Subsequently, based on the voting outcomes of all experts, further discussions were organized to refine and revise the focus issues of the consensus. During the second round, all experts reached a consensus on the recommended opinions by referring to the GRADE grid. The voting protocol stipulated that in case of disagreement, a recommendation or opposition to a specific intervention required at least 50% approval from the participants, while the proportion of participants expressing opposing views should not exceed 20%. Failure to meet this criterion led to the absence of a recommendation. Notably, a strong recommendation demanded at least 70% approval from the participants (13) (Tables 1,2).
Table 1
| Grade | Grade symbol | Current definition |
|---|---|---|
| High | A | We are very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | B | We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | C | Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect |
| Very low | D | We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
GRADE, Grading of Recommendations Assessment, Development and Evaluation.
Table 2
| Recommendation grade | Grade symbol | Current definition |
|---|---|---|
| Strong | 1 | Clearly demonstrate that the advantages of the intervention measures outweigh the disadvantages, or vice versa |
| Weak | 2 | Evidence of mixed quality indicates that the advantages and disadvantages are uncertain or comparable |
GRADE, Grading of Recommendations Assessment, Development and Evaluation.
T2 immune mechanisms and biomarkers in the respiratory system
T2 immunity and T2 inflammation
Based on the involvement of different effector T cells and innate lymphoid cells (ILCs), immunity can be broadly categorized into three types: (I) type 1 immunity, which encompasses Th1 cells, ILC1, natural killer (NK) cells, CD8+ Tc1 cells, and macrophages; (II) T2 immunity, which involves Th2 cells, ILC2, and Tc2 cells; (III) type 3 immunity, which comprises Th17 cells and ILC3. Besides their functions in the usual protective processes of the body, type 1 and type 3 immunity are predominantly associated with autoimmune diseases, while T2 immunity is primarily related to allergic diseases (14,15).
The T2 immune response is primarily driven by GATA-3+ transcription factors expressed in Th2 cells and ILC2. Under normal physiological conditions, it is vital in combating parasite infections and mitigating inflammatory injuries (15). Moreover, epithelial-derived cytokines, such as thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33, can initiate or enhance the T2 immune response, although their functions are not limited to the T2 immune response (1). Excessive activation of T2 immune response can trigger a cascade of inflammatory reactions known as T2 inflammation. This is characterized by the increased synthesis and release of T2 cytokines, such as IL-4, IL-5, and IL-13, leading to tissue infiltration by eosinophils (EOS) and elevated levels of immunoglobulin E (IgE), and ultimately causing various T2 inflammatory diseases (16). Previous studies have reported an enhanced expression of T2 inflammatory factors in bronchoalveolar lavage fluid or other tissues of certain asthma, COPD, and EGPA patients, indicating their association with the T2 immune response (16-23). Additionally, the T2 immune response also contributes to other respiratory system diseases, including ABPA, eosinophilic bronchitis (EB), eosinophilic pneumonia (EP), and bronchiectasis associated with T2 inflammation.
Mechanism of T2 inflammatory respiratory diseases
In T2 inflammation, alarmins can directly activate ILC2, stimulating the production of cytokines such as IL-5, IL-13, and IL-9. These cytokines affect diverse cells, including eosinophils, basophils, mast cells, and epithelial cells, thereby facilitating the initiation of T2 inflammation (24). At the same time, IL-13 enhances the migration of activated dendritic cells to local lymph nodes and, in combination with IL-4, prompts the differentiation of naive T cells (Th0) into Th2 cells. Th2 cells secrete cytokines such as IL-4, IL-5, and IL-13. Along with eosinophil chemotactic factors produced by inflammatory cells such as eotaxin-1 (CCL11), and thymus and activation-regulated chemokine (TARC/CCL17), they jointly promote B-cell class switching and IgE production. Additionally, they recruit inflammatory cells, including eosinophils, to the respiratory tract and lung tissue. T2 cytokines also induce mast cells to generate lipid mediators such as prostaglandin D2 (PGD2). Furthermore, the migration of ILC2 to the lungs is further facilitated, ultimately resulting in the production of T2 cytokines and establishing a mutually influential and synergistic inflammatory loop (25-27) (Figure 1).
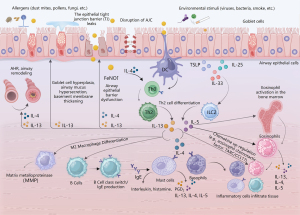
Pathophysiology of T2 inflammatory respiratory disease
The main pathophysiological characteristics of T2 inflammatory respiratory diseases include the impairment of the airway epithelial barrier function, infiltration of inflammatory cells, excessive mucus secretion in the airways, airway remodeling, and airway hyperresponsiveness (AHR) (Table 3). T2 cytokines, specifically IL-4, IL-5, and IL-13, play pivotal roles in the processes above. IL-4 and IL-13 can impair the structure of the epithelial apical junction complex (AJC) (28), while IL-13 disrupts the tight junction barrier of the bronchial epithelium (29). Moreover, IL-4 and IL-13 facilitate the recruitment of eosinophils to the airways and lung tissues. At the same time, IL-5 promotes the differentiation and maturation of eosinophil progenitor cells in the bone marrow, prolongs eosinophil survival in the airways, and activates the release of leukotrienes (LT), and toxic granule proteins such as eosinophil cationic protein (ECP), major essential protein, and eosinophil-derived neurotoxin (30). IL-4 and IL-13 do not directly activate the effector functions of eosinophils but induce eosinophilic airway inflammation by upregulating the expression of vascular cell adhesion molecule-1 (for adhesion) and CC chemokine receptor 3 ligands (for migration) (31).
Table 3
| Pathophysiological characteristics | IL-4 | IL-13 | IL-5 | Role of T2 cytokines in indicated pathophysiological progression |
|---|---|---|---|---|
| Airway epithelial barrier dysfunction | √ | Th2 cell differentiation | ||
| √ | The epithelial TJ barrier leaks | |||
| √ | √ | Disruption of epithelial AJC structures | ||
| Excessive airway mucus | √ | Goblet cell hyperplasia | ||
| √ | Mucociliary dysfunction | |||
| √ | Excessive mucus secretion | |||
| Airway remodeling | √ | Goblet cell hyperplasia | ||
| √ | Collagen deposition | |||
| √ | Airway smooth muscle hyperplasia | |||
| √ | √ | Subepithelial fibrosis | ||
| √ | √ | √ | Eosinophil recruitment and trafficking to tissue | |
| Airway hyperresponsiveness | √ | Increased contractility of airway smooth muscle | ||
| √ | √ | B-cell isotype switching and IgE production | ||
| √ | √ | Mast cell activation and trafficking to tissue | ||
| √ | √ | √ | Participate in neuromodulation | |
| Inflammatory cell infiltration | √ | Eosinophil differentiation and survival | ||
| √ | √ | √ | Eosinophil recruitment and trafficking to tissue |
√, the check marks represent involvement of T2 cytokines IL-4, IL-13, and IL-5 in indicated pathophysiological progression. T2, type 2; IL, interleukin; TJ, tight junction; AJC, apical connecting complex; IgE, immunoglobulin E.
IL-13 is also crucial in promoting goblet cell mucus production and airway remodeling (1). It induces the sustained expression of mucin 5AC (MUC5AC) in human bronchial epithelial cells, thereby impairing mucociliary function, leading to the obstruction of airway mucus clearance and subsequent deterioration in lung function (32-34). IL-13 triggers subepithelial collagen fiber deposition and goblet cell hyperplasia and induces bronchial subepithelial fibrosis via the transforming growth factor-beta (TGF-β) signaling pathway (35,36). Moreover, T2 inflammatory factors promote eosinophil infiltration in the airways, where eosinophils secrete TGF-β (37), stimulating epithelial cells to produce various mediators and ultimately promoting airway smooth muscle proliferation (38), thus contributing to airway remodeling (39).
IL-4 and IL-13 can also induce airway smooth muscle contraction, resulting in airway narrowing and the development of AHR (40,41). Sensory neurons expressing Mas-related G-protein-coupled receptors (Mrgprs) can be stimulated by neuropeptide FF released from mast cells, mediating bronchial smooth muscle contraction and AHR (42,43). In addition, IL-5 can activate sensory neurons in lung tissue, triggering the release of vasoactive intestinal polypeptide, which acts on CD4+ T lymphocytes and ILC2 to amplify the inflammatory response (44). Bronchoconstriction along with increased eosinophils in the airways after allergen challenge enhances the sensitivity of sensory neurons to cause cough hypersensitivity (45).
Recommendation 1
T2 inflammatory respiratory diseases include asthma, a subgroup of COPD, ABPA, EGPA, EB, EP, and a subgroup of non-cystic fibrosis bronchiectasis (1D).
Recommendation 2
The inflammatory T2 response is mediated by Th2 cells, ILC2, and the secretion of T2 cytokines such as IL-4, IL-5, and IL-13, etc. The pathophysiological changes include the disruption of airway epithelial barrier function, infiltration of eosinophils, excessive mucus secretion in the airways, airway wall remodeling, and AHR (1D).
Biomarkers
Currently, biomarkers such as total serum IgE (tIgE), allergen-specific IgE (sIgE), sputum EOS, blood EOS, and fractional exhaled nitric oxide (FeNO) help evaluate T2 inflammation in clinical respiratory diseases. While the Global Initiative for Asthma (GINA) defines T2 inflammatory asthma, other T2 inflammatory respiratory diseases are not as clearly delineated (46).
Assessing tIgE and sIgE levels is crucial for evaluating T2 inflammatory diseases and predicting treatment response to anti-IgE antibody treatment (47). Elevated levels of serum tIgE and sIgE are observed in patients with allergic asthma (48,49). Specific IgE is a major marker for diagnosing allergies, while tIgE is a fundamental reference indicator for allergic asthma and anti-IgE treatment in ABPA. Moreover, in patients with COPD, elevated levels of tIgE and sIgE are associated with exacerbations and lung function decline (50). It is worth noting that IgE production is not only characteristic of allergy but also of infection by parasitic worms, multiple myeloma and other systemic diseases (51,52).
One of the main characteristics of T2 inflammation is an elevation in blood and airway EOS. A sputum EOS count of 3% or more in asthmatic individuals may indicate their responsiveness to inhaled corticosteroids (ICS) (53). In severe asthma, a sputum EOS count of 2% or above is associated with persistent airflow limitation and suboptimal asthma control (54). Among stable COPD patients, an increased sputum EOS count can predict a higher risk of future exacerbations and is correlated with a positive response to ICS (55). Additionally, studies demonstrate that a sputum EOS count of 2% or higher is significantly associated with diminished lung function and emphysema severity in COPD patients (56).
There is a correlation between blood EOS count and sputum EOS in patients with asthma (57), which can predict outcomes such as asthma exacerbations and lung function decline (58,59). Since 2019, GINA has included a blood EOS count of 150/µL or higher as one of the criteria for diagnosing T2 asthma (46). It is also recommended as a biomarker for treating severe asthma with anti-IL-5, anti-IL-5R, and anti-IL-4R antibodies (46). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends considering the use of blood EOS count (>300 cells/µL) as a reference for ICS treatment in COPD patients (60). Additionally, it is crucial to rule out parasitic infections, tumors, and rheumatic immune diseases when evaluating an elevated EOS count.
FeNO is a non-invasive biomarker for T2 asthma, featuring simplicity, rapidity, and good repeatability. GINA suggests a FeNO level ≥20 ppb as a criterion for diagnosing T2 asthma (46). Studies have revealed that FeNO levels exceeding 47 ppb correlate with increased airway eosinophils and steroid response, and are predictive factors for asthma exacerbations (61,62). GINA recommends FeNO as a biomarker for predicting the response to anti-IL-4R (46). FeNO also emerges as a potential biomarker for predicting increased airway eosinophils and corticosteroid responsiveness in COPD, and its presence is associated with an increased risk of COPD exacerbations (63,64). FeNO is more precise than blood eosinophil count in forecasting severe COPD exacerbations (65). Combining these two biomarkers enhances the identification of moderate to severe exacerbations of COPD.
Recommendation 3
Serum tIgE, sIgE, blood and sputum EOS, and FeNO can be used to assess T2 inflammation and predict treatment response to biologics. These tests should be performed before starting and adjusting treatment strategies (1D).
Recommendation 4
Elevated levels of serum tIgE and blood eosinophil count should exclude the potential impact of parasitic infections and/or other clinical conditions (1C).
Overview of biological therapies
Biological therapies for T2 inflammation are precise treatments for T2 inflammatory respiratory diseases and their comorbidities. This approach specifically blocks or inhibits target molecules in the inflammatory pathways of these diseases to suppress the inflammatory responses mediated by these pathways and maximize treatment safety.
Currently, biological therapies approved by China National Medical Products Administration (NMPA), the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of asthma and other T2 inflammatory diseases include anti-IgE antibody (omalizumab), anti-IL-4 receptor α (anti-IL-4Rα) antibody (dupilumab), anti-IL-5 antibody (mepolizumab) and anti-IL-5 receptor antibody (benralizumab). Both FDA and EMA have approved anti-TSLP antibodies (tezepelumab) but not by NMPA. For the specific approval status, indications, and research progress of these biologics, refer to Table 4.
Table 4
| Biologics | Omalizumab (anti-IgE) | Dupilumab (anti-IL-4R) | Mepolizumab (anti-IL-5) | Benralizumab (anti-IL-5R) | Tezepelumab (anti-TSLP) |
|---|---|---|---|---|---|
| Mechanism | The recombinant humanized IgG monoclonal antibody binds to free IgE, reduces the expression of high affinity FcγRI receptors, and inhibits the IgE signaling pathway (66) | A fully human monoclonal antibody that inhibits both IL-4 and IL-13 signaling pathways by binding to IL-4Rα (67) | The humanized IgG1/κ monoclonal antibody, sourced from mice, primarily binds tightly to IL-5 and suppresses the IL-5 signaling pathway (68) | The humanized monoclonal antibody binds to IL-5R, inhibiting the IL-5 signaling pathway and also inducing EOS apoptosis by acting on NK cells (69) | A fully human monoclonal antibody that binds to TSLP, blocks the formation of a complex with TSLP receptor and IL-7R, thereby inhibiting the TSLP signaling pathway (70) |
| Asthma indications | FDA: patients aged ≥6 years with moderate to severe persistent allergic asthma. EMA: patients aged ≥6 years with severe persistent allergic asthma | FDA: patients aged ≥6 years with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma. EMA: patients aged ≥6 years with severe asthma and type 2 inflammation (elevated FeNO/EOS) | FDA: patients aged ≥6 years with severe asthma and with an eosinophilic phenotype. EMA: patients aged ≥6 years with severe asthma and with an eosinophilic phenotype | FDA: patients aged ≥6 years with severe asthma and with an eosinophilic phenotype. EMA: patients aged ≥18 years with severe refractory asthma and with an eosinophilic phenotype | FDA: patients aged ≥12 years with severe asthma. EMA: patients aged ≥12 years with severe asthma |
| NMPA: patients aged ≥6 years with moderate to severe allergic asthma ICS/LABA conventional treatment for ≥3 months | NMPA: asthma aged ≥12 years who are inadequately controlled under regular treatment with medium-high dose ICS plus another controller and with type 2 inflammation, or oral corticosteroid-dependent asthma | NMPA: patients aged ≥12 years with severe asthma and with an eosinophilic phenotype | NMPA: patients aged ≥12 years with severe asthma and with an eosinophilic phenotype | ||
| COPD indications | Phase II withdrawal due to lack of eligible subjects with elevated IgE in COPD | FDA: adults with inadequately controlled COPD and an eosinophilic phenotype. EMA: adults with uncontrolled COPD characterized by raised blood eosinophils on a combination of an ICS, an LABA, and a LAMA, or a combination of an LABA and a LAMA if ICS is not appropriate. NMPA: the same with EMA | COPD with elevated EOS count. Phase III | Patients with a history of exacerbations of COPD. Phase III | Phase IIa has been completed but not yet been released |
| EGPA indications | Unavailable | Unavailable | FDA: patients with EGPA, aged ≥18 years. EMA: patients with EGPA that has relapsed and is refractory to other medications, aged ≥6 years. NMPA: the same with FDA | FDA: patients with EGPA, aged ≥18 years | Unavailable |
| ABPA indications | Unavailable | Phase II recruitment | Unavailable | Unavailable | Unavailable |
ABPA, allergic bronchopulmonary aspergillosis; COPD, chronic obstructive pulmonary disease; EGPA, eosinophilic granulomatosis with polyangiitis; EMA, European Medicines Agency; FDA, Food and Drug Administration; FeNO, fractional exhaled nitric oxide; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL, interleukin; TSLP, thymic stromal lymphopoietin; LABA, long-acting beta-2 receptor agonist; LAMA, long-acting muscarinic anticholinergics; NMPA, China National Medical Products Administration; T2, type 2.
T2 inflammatory respiratory disease
Asthma
Asthma manifests as chronic airway inflammation and is clinically characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and cough. These symptoms vary in timing and intensity and are often accompanied by variable expiratory airflow limitation, which may persist in some patients (46).
As a multifaceted and heterogeneous disease, asthma has various clinical features, that are driven by different underlying pathophysiological mechanisms. Based on patients’ immune-inflammatory profiles, asthma can be categorized into two main endotypes: T2 asthma and non-T2 asthma (66-71) (Figure 2). According to the inflammatory classification, T2 asthma includes allergic asthma, eosinophilic asthma, and other mixed inflammatory endotypes/phenotypes (elevated T2 biomarkers such as FeNO) (71).
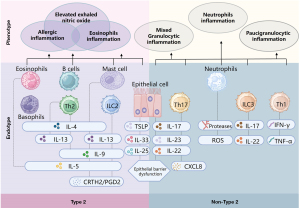
The exact prevalence of T2 asthma is unclear. A pathological study using the expression of T2-associated transcripts in bronchial brushings indicated that approximately 50% of mild to moderate asthma cases can be classified as T2 asthma (72). The prevalence of this endotype is even higher among severe asthma cases (18,73). In China, around 56–76% of adults with severe asthma and 80% of children with asthma are classified as T2 asthma (74,75).
Severe asthma is “asthma which requires treatment with high dose ICS plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy” (76). Uncontrolled asthma defined as at least one of the following (77): (I) poor symptom control: Asthma Control Questionnaire (ACQ) consistently ≥1.5, Asthma Control Test (ACT) <20 (or “not well controlled” by NAEPP/GINA guidelines). (II) Frequent severe exacerbations: two or more bursts of systemic corticosteroid (3 days each) in the previous year. (III) Serious exacerbations: at least one hospitalization, intensive care unit (ICU) stay or mechanical ventilation in the previous year.
Role and assessment of T2 inflammation in asthma
T2 asthma is driven by Th2 cells and ILC2 (78). The detailed mechanisms and key pathophysiological features are respectively shown in Figure 1 and Table 3. Elevated levels of downstream biomarkers such as FeNO, serum IgE, and EOS in sputum and blood are also associated with this asthma endotype (78).
According to the latest GINA report, T2 asthma can be identified by meeting any one of the following criteria (46): (I) blood EOS count ≥150/µL; (II) FeNO ≥20 ppb; (III) sputum EOS count ≥2%; (IV) asthma is clinically allergen-driven. Individuals taking oral corticosteroid (OCS) should discontinue their use or maintain the lowest dose for 1–2 weeks before re-testing blood EOS count, serum tIgE or sIgE, and FeNO for reassessment because of the potential suppressive effect of OCS on these parameters.
Biological treatment of T2 asthma
Typically, asthma medications are categorized into controller medications and reliever medications. Controller medications commonly include ICS, leukotriene modifiers, inhaled long-acting beta-2-agonists (LABA), inhaled long-acting muscarinic anticholinergics (LAMA), sustained-release theophylline, or sodium cromoglycate. Reliever medications commonly comprise inhaled short-acting beta2-agonists, inhaled short-acting anticholinergics, short-acting theophylline, and systemic steroids. Recently, GINA 2024 recommended low-dose ICS-formoterol being taken as maintenance-and-reliever therapy rather than as-needed-only (46). Additionally, for severe asthma unresponsive to medium to high-dose ICS/LABA therapy, LAMA, and biologics are the main additional therapeutic choices (79).
The biologics currently used for treating T2 asthma include omalizumab, dupilumab, mepolizumab, benralizumab, and tezepelumab (46). The phase 3 clinical studies conducted have demonstrated the effectiveness of omalizumab (80-84), dupilumab (85-89), mepolizumab (90-93), benralizumab (94-98) and tezepelumab (99-101) in reducing acute exacerbations, improving lung function, and controlling asthma in patients with T2 asthma. Furthermore, they have shown the ability to enhance the quality of life and exhibit favorable tolerability and safety profiles (46) (Table 5).
Table 5
| Target | Biological agents | Indications | Eligibility | Clinical efficacy |
|---|---|---|---|---|
| IgE | Omalizumab (80-84) | Patients aged ≥6 years with moderate to severe allergic asthma | Positive on skin prick testing or sIgE. tIgE and weight within dosage range. Exacerbations in last year | Reduce asthma exacerbations; improve lung function and asthma control; enhance the quality of life; reduce OCS use |
| IL-4R | Dupilumab (85-89) | Patients aged ≥6 years with moderate-severe type 2 uncontrol asthma, or requiring treatment with maintenance OCS | Exacerbations in last year. Blood EOS ≥150/μL and ≤1,500/μL. Or FeNO ≥25 ppb, or taking maintenance OCS | Reduce asthma exacerbations; improve lung function; improve asthma control; enhance the quality of life; reduce OCS use; achieve sustained clinical benefits through long-term treatment (up to 96 weeks) |
| IL-5 | Mepolizumab (90-93) | Patients aged ≥6 years with severe eosinophilic asthma | Exacerbations in last year. Blood EOS ≥150/μL | Reduce asthma exacerbations; improve lung function; improve asthma control and the quality of life; reduce OCS use |
| IL-5R | Benralizumab (94-98) | Patients aged ≥12 years with severe eosinophilic asthma | Exacerbations in last year. Blood EOS ≥300/μL | Reduce asthma exacerbations; reduce OCS use; enhance the quality of life; achieve sustained clinical benefits through long-term treatment (up to 5 years) |
| TSLP | Tezepelumab (99-101) | Patients aged ≥12 years with severe asthma | Exacerbations in last year | Reduce asthma severe exacerbations; improve quality of life, lung function; achieve sustained clinical benefits through long-term treatment (up to 104 weeks) |
EOS, eosinophil; FeNO, fractional exhaled nitric oxide; OCS, oral corticosteroids; IgE immunoglobulin E; sIgE, allergen-specific IgE; tIgE, total serum IgE; IL, interleukin; TSLP, thymic stromal lymphopoietin.
Recommendation 5
For patients with uncontrolled moderate to severe allergic asthma, anti-IgE (omalizumab) is recommended as add-on treatment (1A).
Recommendation 6
For patients with moderate to severe T2/eosinophilic asthma, or requiring maintenance OCS, anti-IL-4Rα (dupilumab) is recommended as add-on treatment (1A).
Recommendation 7
For patients with severe eosinophilic asthma, anti-IL-5/5Rα (mepolizumab or benralizumab) is recommended as add-on treatment (1A).
Recommendation 8
For patients with uncontrolled severe asthma, anti-TSLP (tezepelumab) is recommended as add-on treatment (1A).
COPD
COPD is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production, and/or exacerbations) due to abnormalities in airways (bronchitis, bronchiolitis) and/or alveoli (emphysema), causing persistent and often progressive airway obstruction (102). COPD can progress to cor pulmonale and respiratory failure, leading to increased morbidity and mortality rates. COPD results from gene (G)-environment (E) interactions throughout an individual’s lifetime (T) (102), which can damage the lungs and/or alter their normal development/aging processes. The main exposures are tobacco smoking and inhalation of toxic particles and gases from both household and outdoor sources (102). Between 1990 and 2019, 46.0% of the disability-adjusted life years due to COPD were caused by smoking across 204 countries and regions worldwide (103). Meanwhile, the odds ratio of risk factors for COPD smoking exposure 20 pack-years or more in China was 1.95 [95% confidence interval (CI): 1.53–2.47] between 2012 and 2015 (104).
Role and assessment of T2 inflammation in COPD
The inflammatory response in COPD is diverse (105), with a subgroup (up to 40%) of patients having a predominant T2 inflammatory response (106). When airway epithelial cells are stimulated by harmful stimuli such as smoke, allergens, bacteria or viruses, T2 immune response is activated (107). A persistent excessive response leads to the development of COPD (107). COPD with T2 inflammation typically exhibits increased blood EOS, elevated FeNO, higher rates of exacerbation, greater bronchodilator reversibility, and a higher prevalence of asthma-like symptoms (65,108). However, standardized diagnostic criteria for COPD with T2 Inflammation have yet to be established, and most studies rely on elevated EOS in blood and/or sputum. Nearly 37% of COPD patients exhibit persistently elevated blood EOS (≥2%) (109), and approximately 15–33% show persistent blood EOS ≥300/µL (106,109).
Biological therapy of COPD
To date, various biologics have been explored in treating COPD with T2 inflammation, and only anti-IL-4Rα (dupilumab) has displayed promising results. Two phase 3 clinical trials, namely the BOREAS study and the NOTUS study, investigating the efficacy and safety of dupilumab in moderate to severe COPD patients with T2 inflammation (blood EOS ≥300 cells/µL) and a history of relevant exacerbations have been completed. The BOREAS (110) and NOTUS (111) studies demonstrated a 30% and 34% reduction, in the annual rate of moderate or severe exacerbations over 52-week treatment with dupilumab compared to placebo. Moreover, dupilumab significantly improved forced expiratory volume in 1 second (FEV1), symptoms, and health-related quality of life compared to placebo, and these benefits persisted up to 52 weeks (110,111). Recently, a real-world study from China further indicated that dupilumab combined with triple inhaled therapy can enhance pulmonary function, alleviate allergic symptoms, attenuate T2 inflammatory biomarker levels, and improve quality of life of COPD patients with T2 inflammation (112).
Several large phase 3 studies have investigated the efficacy of anti-IL-5 and anti-IL-5R such as mepolizumab and benralizumab in patients with moderate to severe COPD (113,114). However, these two biologics were not consistently shown to have lower annual rates of moderate or severe exacerbations compared to placebo among patients with COPD and an eosinophilic phenotype (113,114). The efficacy and safety of monoclonal antibody against the upstream alarmin IL-33 and TSLP, such as itepekimab, tozorakimab, astegolimab, and tezepelumab, in COPD patients are currently under investigation (115,116).
Recommendation 9
For patients with uncontrolled COPD who still have exacerbation risk despite being treated with ICS + LABA + LAMA, and having T2 inflammation (e.g., blood eosinophil ≥300/µL), anti-IL-4Rα (dupilumab) is recommended as add-on treatment (1A).
ABPA
ABPA is an allergic lung disease caused by the colonization of Aspergillus fumigatus (AF) spores in the airways of susceptible individuals. Its main clinical manifestations include asthma-like symptoms and bronchiectasis, frequently accompanied by pulmonary imaging findings of wandering pulmonary shadows or mucus obstruction (117,118). ABPA is often misdiagnosed as bronchial asthma. Retrospective studies indicate that 93% of Chinese ABPA patients have asthma symptoms (119).
Role and assessment of T2 inflammation in ABPA
The inhalation of fungal conidia-triggered T2 immune response is regarded as the primary pathogenesis of ABPA. Its main pathophysiological features include increased production of T2 cytokines IL-4, IL-5 and IL-13, leading to elevated serum tIgE level, the synthesis of AF sIgE and IgG, goblet cell metaplasia, mucus deposition in the airways, and aggregation of Th2 cells and EOS in the lungs (120). Persistent inflammation and tissue damage contribute to the development of bronchiectasis, which may progress to pulmonary fibrosis and respiratory failure (121).
Elevated levels of T2 inflammation biomarkers serve as important diagnostic criteria for ABPA. The critical values are serum AF sIgE ≥0.35 kU/L, tIgE ≥1,000 U/mL, and EOS count ≥0.5×109/L (117). A recent Chinese study identified the optimal diagnostic efficiency with a critical AF sIgE of 4.108 kU/L and a critical EOS count of 0.815×109/L (122).
Biological therapy of ABPA
The principles of ABPA therapy are to control inflammation, minimize exacerbations, reducing fungal burden and prevent the progression of lung damage. The standard treatment comprises systemic corticosteroids and antifungal therapy. Currently, OCS is preferred for managing ABPA, while ICS is commonly used to alleviate asthma symptoms in ABPA patients (117,121).
Biological therapies have been reported as adjunctive treatments for ABPA. Recently, a comprehensive systematic review and meta-analysis has been conducted, including all available studies measuring the effects of four biologics, omalizumab, dupilumab, mepolizumab, and benralizumab, for treating ABPA (123). Among these biologics, omalizumab has been the most extensively studied. A small-scale randomized controlled trial (RCT) study has shown that omalizumab can significantly reduce exacerbation rates and OCS dosages and improve lung function in the treatment of ABPA (124). The other three biologics, mainly reported in case series or case reports, all demonstrated clinical benefits in ABPA patients (123). However, due to limited evidence in ABPA patients, the efficacy and safety of biologics need further validation through large-scale RCTs.
Recommendation 10
Anti-IgE (omalizumab) are recommended as an optional treatment strategy for ABPA, considering the availability and affordability (1B).
EGPA
EGPA is a rare autoimmune disease that affects multiple body systems. Its main manifestations are increased EOS in blood and tissue and infiltration and necrotizing granulomatous inflammation of small and medium-sized vessels. Except for serum antineutrophil cytoplasmic autoantibodies (ANCA), EOS is also a key player in the disease’s development. Nearly all EGPA patients have an allergic background, with over 96% presenting with asthma and/or sinusitis. In the advanced stages, EGPA can involve multiple tissues and organs throughout the body (125,126).
Role and assessment of T2 inflammation in EGPA
The main driver of EGPA is CD4+ T cells, especially those of the Th2 subtype. T2 cytokines like IL-4, IL-5 and IL-13 are pivotal in the tissue inflammatory response induced by EGPA (126,127). It is estimated that only 40% to 60% of EGPA patients are ANCA-positive. ANCA-positive patients may exhibit more vascular disease features, such as purpura, neuropathy, pulmonary-renal syndrome, while ANCA-negative patients may present more EOS-driven symptoms, like pulmonary infiltrates and cardiomyopathy (127,128).
Biological therapy of EGPA
In current clinical practice, EGPA is commonly treated with OCS and immunosuppressants. Although OCS can effectively relieve symptoms, EGPA tends to relapse when OCS is tapered or discontinued (125). With the wide application of biologics, several studies have demonstrated the efficacy of therapies targeting T2 inflammatory pathways. A phase 3 clinical trial (129) has shown that mepolizumab can improve the remission rates of EGPA, reduce recurrence, and decrease OCS dosages compared to placebo, leading to its FDA approval for EGPA treatment. Recently, an evidence-based guideline recommends adding mepolizumab in combination with glucocorticoids for remission maintenance in patients with severe EGPA or those with relapsing-refractory EGPA without organ- or life-threatening manifestations (130). An observational study (131) suggests that a regimen based on sequential rituximab (a monoclonal antibody targeting CD20, a protein found on the surface of B cells) and mepolizumab might be effective to induce and maintain remission of both systemic and respiratory EGPA manifestations. A retrospective cohort study suggests that benralizumab could be an effective treatment for EGPA in real-life clinical practice (132). Recently, a head-to-head phase 3 trial (MANDARA) evaluated the efficacy and safety of benralizumab in comparison to mepolizumab and revealed that benralizumab was non-inferior to mepolizumab for the induction of remission in patients with relapsing or refractory EGPA (133).
Recommendation 11
For patients with non-severe EGPA undergoing immunosuppressive therapy or on low-dose oral glucocorticoids and experiencing non-severe recurrence, it is recommended to add mepolizumab or benralizumab as a combination therapy (1B).
EB
EB is a common underlying cause of chronic cough, usually triggered by exposure to dust, oil smoke and other harmful stimuli (134). EB presents with chronic cough and sputum eosinophilia, but without the abnormalities of airway function seen in asthma (135). Some patients may present AR (136), while their respiratory system physical examination usually reveals no significant abnormalities, the chest X-ray and pulmonary ventilation function are normal, and the bronchial provocation test is negative (134).
Role and assessment of T2 inflammation in EB
EB is a disease driven by T2 inflammation. Besides the increased level of airway EOS, airway mucosal biopsies in EB patients also reveal elevated levels of T lymphocytes, mast cells and ILC2, as well as increased levels of inflammatory mediators and T2 cytokines such as histamine, LT, IL-4, IL-5 and ECP. These factors contribute to airway mucus secretion, plasma exudation, and inflammatory cell infiltration (137,138). A sputum EOS count of ≥2.5% is a necessary diagnostic criterion for EB (139). Additionally, a FeNO level of ≥32.5 ppb is helpful for the clinical diagnosis of EB (140).
Biological therapy of EB
The primary treatment for EB is anti-inflammatory therapy, commonly employing ICS (141), and long-term OCS therapy is seldom necessary (142). Brightling et al. have also described a patient with EB who developed fixed airflow obstruction in association with prolonged uncontrolled eosinophilic airway inflammation (143). Others have speculated that EB is an early stage in the development of an asthma phenotype (144). Currently, biologics for EB treatment are still in the clinical trial phase. Early studies of mepolizumab (NCT00292877) (145) have demonstrated its efficacy in controlling specific clinical symptoms of EB, offering a novel approach to treat EB. However, no data is available on the recurrence of EB after drug discontinuation, and further long-term studies are required to assess the clinical effect of biological therapy.
Recommendation 12
Biological therapy is not recommended for EB patients due to insufficient RCT evidence and limited real-world studies (1D).
EP
EP is characterized by substantial infiltration of EOS in the lung parenchyma or alveoli and is classified into acute EP (AEP) and chronic EP (CEP) (146). The onset of AEP is rapid, typically within a few days to a few weeks. The main clinical manifestations of AEP are acute fever and dyspnea, which may be complicated by respiratory failure and the rapid emergence of pulmonary shadows. The age of onset of most AEP patients is about 20 years old, which is often related to smoking (147). Blood EOS levels may be normal in AEP. Early diagnosis and timely treatment can lead to rapid remission of symptoms and rare relapse (146-150). The onset of CEP is insidious, chronic, and progressive, featuring common symptoms such as cough and dyspnea, with respiratory failure being rare. Most CEP patients are between 30 and 50 years old, and less than 10% of CEP patients are smokers (149). Blood EOS can increase significantly in the early stage of CEP, and nearly one-third of patients may exhibit specific imaging manifestations known as the “pulmonary edema reversal sign”. Approximately two-thirds of patients have a history of allergic asthma and respond well to glucocorticoids but are prone to relapse (150,151).
Role and assessment of T2 inflammation in EP
Existing evidence suggests that AEP is a disease related to T2 inflammation. After inhalation or exposure to tobacco and other antigens, alveolar or epithelial damage triggers inflammatory signals. It enhances the secretion of IL-33, IL-25 and TSLP, thereby activating a T2 inflammatory response and leading to the activation and recruitment of EOS in the lung (146). Because most patients with AEP have no peripheral eosinophilia, detailed disease history and computed tomography images comprise cues to suspect AEP (146).
Biological therapy of EP
Currently, OCS remains the primary treatment for EP and is usually adequate for most patients, with treatment durations typically ranging from 6 to 12 months (146). However, over 50% of CEP patients have relapses and require recurrent OCS therapy (146). Considering the potential serious adverse reactions associated with the long-term use of OCS, alternative treatments involving ICS and biological therapies are being evaluated. However, most of these alternative treatments are mainly supported by case reports (152,153), and solid evidence is still lacking. Further RCT and real-world studies are required to investigate biologics’ indications, effectiveness, and safety in CEP treatment.
Recommendation 13
Biological therapy is not recommended for EP patients due to insufficient RCT evidence and limited real-world studies (1D).
Non-cystic fibrosis bronchiectasis
Non-cystic fibrosis bronchiectasis is a recurrent suppurative infection disease caused by various factors, resulting in repeated damage and/or obstruction of small and medium bronchi, structural destruction of bronchial walls, and persistent bronchiectasis. Its clinical manifestations include chronic cough, expectoration, intermittent hemoptysis, and possibly dyspnea or respiratory failure (154). Traditionally viewed as a neutrophil-dominated chronic airway inflammatory disorder, bronchiectasis has recently garnered attention for the potential of eosinophils involved in its pathogenesis. A European study indicated approximately 30% of patients exhibit sputum EOS ≥3% (155), while a European multi-cohort study (156) demonstrated that 22.6% had blood EOS levels ≥300/µL. By defining eosinophilic bronchiectasis with blood EOS ≥300/µL or FeNO ≥25 ppb as T2 inflammatory bronchiectasis, roughly 31% of patients have been classified under this subtype. These patients typically suffer from more severe dyspnea, worse lung function, and poorer quality of life (157).
Role and assessment of T2 inflammation in non-cystic fibrosis bronchiectasis
Eosinophilic bronchiectasis patients typically exhibited elevated FeNO and sputum IL-13 levels, and enhanced responsiveness to bronchodilators (155). Blood EOS ≥300/µL in bronchiectasis patients has been associated with Streptococcus and Pseudomonas infections (155). Pseudomonas aeruginosa (PA), a prevalent opportunistic pathogen, secretes redox-active exotoxins like pyocin, inhibiting Th1 response and promoting Th2-driven inflammation. Additionally, PA-secreted toxins, such as elastase B, may activate the expression of epithelial amphiregulin, contributing to T2 inflammation (158,159). These findings suggest that T2 inflammation is important in bronchiectasis onset and progression. Furthermore, bronchiectasis frequently co-occurs with diseases marked by enhanced T2 inflammation, including asthma, ABPA, and CRSwNP (159). However, a unified diagnostic criterion for T2 inflammatory bronchiectasis remains elusive.
Biological therapy of non-cystic fibrosis bronchiectasis
The application of biologic therapy in bronchiectasis remains in the nascent stages of exploration. Currently, multiple T2 inflammatory biologics, omalizumab,dupilumab, mepolizumab, benralizumab, and reslizumab, are being evaluated for potential therapeutic benefits in managing bronchiectasis. Though several case reports and retrospective studies hinted at their ability to reduce acute exacerbations, decrease blood and sputum EOS levels, and minimize OCS dosages or shorten their durations in asthmatic patients with bronchiectasis, comprehensive large-scale studies are necessary to confirm these observations (160,161). Specifically, biological therapies for bronchiectasis alone are underrepresented in current research, with a notable exception being the ongoing MAHALE study (NCT05006573) investigating the efficacy of benralizumab in non-cystic fibrosis bronchiectasis. The results of this study remain unpublished (162).
Preliminary indications indicate a therapeutic promise for biological therapies in bronchiectasis, yet several hurdles impede their widespread implementation. Establishing a standardized classification system for bronchiectasis-associated airway inflammation is paramount to optimizing tailored therapeutic strategies. The current lack of such a framework poses challenges in selecting the most suitable treatment approach. Furthermore, the intricate pathogenesis of bronchiectasis remains largely elusive, underscoring the need for continued exploration into the intricacies of the associated airway inflammation, particularly the T2 predisposition.
Recommendation 14
Biological therapy is not recommended for non-cystic fibrosis bronchiectasis patients due to the lack of current RCT evidence and limited real-world studies (1D).
Comorbidities associated with T2 inflammatory respiratory disease
Epidemiology and mechanisms
T2 inflammatory respiratory diseases frequently coexist with a myriad of other T2 inflammatory conditions, including both lower (asthma, COPD and ABPA) and upper (AR and CRSwNP) respiratory disorders, nonsteroidal anti-inflammatory drugs (NSAID)-exacerbated respiratory disease (NERD), aspirin-exacerbated respiratory disease, and non-respiratory co-morbidities such as atopic dermatitis (AD), eosinophilic esophagitis (EOE), ocular diseases, other gastrointestinal disorders, food allergies and others (163). Patients with T2 inflammatory respiratory disease and comorbidities experience significantly intensified symptoms, more frequent exacerbations, prolonged hospitalization, and escalated healthcare cost compared to those without comorbidities (164,165). This heightened disease burden is further compounded by an increased reliance on OCS (166,167). Surveys indicate that over 59% of patients harbor more than two comorbidities, which significantly diminish their quality-of-life regardless of severity (168). The prevailing evidence underscores the pivotal role of T2 inflammation in these respiratory diseases. Patients may manifest one or more clinical features of T2 inflammation, including elevated serum levels of tIgE and sIgE, and increased EOS in blood or tissue (1).
Biological therapy on the comorbidity of T2 inflammatory respiratory diseases
Currently, large-scale clinical studies on the comorbidity of T2 inflammatory respiratory diseases are scarce. Most studies have predominantly focused on asthma comorbidities, such as asthma co-occurring with AR, CRSwNP, AD, and COPD. Among these, the asthma-CRSwNP interplay has garnered attention. Real-world investigation into the co-existence of CRSwNP and asthma has demonstrated the effect of omalizumab, dupilumab, mepolizumab, and benralizumab to alleviate both asthma and nasal symptoms (169-173). Statistically significant changes were achieved after 4- or 6 months treatment (170,171). Furthermore, a post-hoc analysis across five phase 3 studies revealed the significant impact of dupilumab in improving asthma, sinusitis, and AD symptoms (174). The analysis also mentions that improvements with dupilumab are rapid and continue beyond the first months of treatment (174). Similarly, in asthma coexisting with AR, a post-hoc analysis of phase 3 study underscored dupilumab’s effectiveness in reducing asthma exacerbations, enhancing lung function, and improving the quality-of-life scores related to rhinoconjunctivitis (175). In addition, in cases of asthma comorbid with COPD, a single-center retrospective study pointed to mepolizumab’s benefits in reducing EOS, exacerbation frequency, OCS dosages, and enhancing lung function after 6 months treatment (176).
Strategies to block T2 inflammatory mediators correlate with favorable outcomes across various T2 inflammatory diseases. In particular, omalizumab has been proven effective in asthma, CRSwNP, and chronic spontaneous urticaria. Dupilumab, on the other hand, exhibits therapeutic value in asthma, COPD, CRSwNP, AD, and EOE (177). Mepolizumab and benralizumab similarly show efficacy in asthma, CRSwNP, and EGPA (129,170,171). Thus, in managing multi-system T2 inflammatory respiratory diseases and non-respiratory co-morbidities, it is imperative to consider the involvement of different systems that can guide the selection of the most appropriate biological therapy.
Multidisciplinary management of comorbidities
Managing T2 inflammation-related comorbidities often requires a multidisciplinary approach. Implementing a multi-disciplinary treatment model is recommended to effectively manage these comorbidities of T2 inflammatory respiratory diseases and formulate personalized diagnosis and treatment plans for patients. Multidisciplinary management also can improve patients’ comprehension of their conditions, promote treatment adherence, enhance self-management skills, and ultimately boosting their quality of life. Establishing multidisciplinary care teams also guarantees patient-centered care, optimizes direct and indirect outcomes, reduces costs, and facilitates more appropriate treatment decisions (178,179). However, the specific model and future development directions of multidisciplinary management in China still require further exploration and improvement (Figure 3).
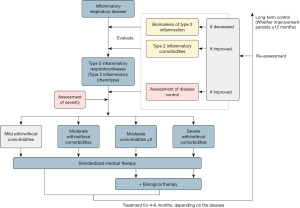
Recommendation 15
In treating T2 inflammatory respiratory diseases, it is recommended to consider a multidisciplinary evaluation of related comorbidities based on the patient’s clinical manifestations. This evaluation should include assessments of comorbidities, biomarkers, the impact on quality of life, and the utilization of currently-available medications (1D).
Recommendation 16
When selecting T2 inflammation-targeted therapies, the preferred initial biologics should target the disease that most significantly impacts the quality of life and be consistent with the approved indications. The efficacy of biologics should be evaluated 4 to 6 months after treatment by assessing clinical outcomes (1A).
Perspectives
This is the first Chinese expert consensus aimed at guiding Chinese clinicians in the treatment and management of T2 inflammatory respiratory diseases. Despite progress, there are limitations with current biomarkers, and no single biologics can effectively cure all T2 inflammatory disorders. However, ongoing research on pathophysiological mechanisms and treatment approaches offers hope for developing new biomarkers. These emerging markers aim to improve the accuracy of diagnosis and management of T2 inflammatory respiratory diseases. Recent studies have identified several potential biomarkers for T2 inflammation, including dipeptidyl peptidase-4 (DPP-4), volatile organic compounds in exhaled breath, the percentage and count of ILC2 in peripheral blood, ECP, eosinophil derived neurotoxin (EDN), CCL11, and mas-related G protein-coupled receptor X2 (MRGPRX2), as well as protein S chemokines or inflammatory mediators (180-184). RNA quantification of IL-4, IL-5, and IL-13 gene expression in induced sputum may also help identify T2 inflammation, more precisely (18,185).
Other biologics targeting T2 inflammation are being investigated. These may include anti-IL-9, anti-IL-25, anti-IL-33, anti-TSLP, bispecific anti-IL-13/TSLP (186), and other antibodies. Tezepelumab is an investigational human IgG2 monoclonal antibody that binds to TSLP, which is now available in North America and Europe. Other types of biological agents include prostaglandin D2 receptor (DP2/CRTH2) antagonists, anti-GATA C3 antibodies, JAK inhibitors, BTK inhibitors and anti-OX40L antibodies (187-190). However, given that T2 inflammatory respiratory disease is systemic, with persistent abnormal levels of inflammatory cells and factors as the main driver, questions arise as to whether T2 inflammation-targeted agents could replace current basic respiratory therapy as the primary systemic treatment. Also, can we target a broader spectrum of mild-to-moderate patients instead of only those with severe conditions? These questions require further exploration, but individualized and precise treatment will inevitably shape the future development direction in this field.
Conclusions
T2 inflammatory respiratory diseases are characterized by T2 inflammation. T2 inflammatory respiratory diseases include asthma, a subgroup of COPD, ABPA, EGPA, EB, EP, and a subgroup of non-cystic fibrosis bronchiectasis. T2 inflammation-targeted therapies show high potent in treatment for T2 inflammatory respiratory diseases and their comorbidities. However, there is still a lack of high-quality studies to evaluate the clinical effects. Moreover, biologics that can effectively treat all T2 inflammatory disorders need to be explored.
Acknowledgments
We acknowledge the below experts for their contribution to the consensus—Consultant in Chief: Prof. Nan Shan Zhong from the First Affiliated Hospital of Guangzhou Medical University. International Consultants: Prof. Kian Fan Chung from Imperial College London, United Kingdom; Professor Gary Wing-Kin Wong from Chinese University of Hong Kong, Hong Kong SAR, China; Prof. Paul M. O’Byrne from McMaster University, Canada. Consultants: Prof. Yinshi Guo from the Department of Allergy, Renji Hospital of Shanghai Jiao Tong University School of Medicine. Prof. Jie Shao from the Department of Pediatrics, Ruijin Hospital of Shanghai Jiao Tong University School of Medicine. Prof. Limin Zhao from the Department of Respiratory Medicine, Henan Provincial People’s Hospital. Prof. Chun Chang from the Department of Respiratory Medicine, Peking University Third Hospital. Prof. Haijin Zhao from the Department of Respiratory Medicine, Nanfang Hospital of Southern Medical University. Prof. Chuangli Hao from the Department of Respiratory Medicine, Children’s Hospital of Soochow University. Prof. Juntao Feng from the Department of Respiratory Medicine, Xiangya Hospital of Central South University. Prof. Yuqing Wang from the Department of Respiratory Medicine, Children’s Hospital of Soochow University. Prof. Xinming Su from the Department of Respiratory Medicine, the First Hospital of China Medical University. Prof. Yadong Gao from the Department of Allergy, the First Affiliated Hospital of Zhejiang University School of Medicine. Methodology Consultant: Dr. Jiang Mei from the First Affiliated Hospital of Guangzhou Medical University.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2092/prf
Funding: This work was supported by grants of
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2092/coif). R.C. serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gandhi NA, Bennett BL, Graham NM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016;15:35-50. [Crossref] [PubMed]
- Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet 2018;391:783-800. [Crossref] [PubMed]
- Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022;10:447-58. [Crossref] [PubMed]
- Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013;51:361-70. [Crossref] [PubMed]
- Jakes RW, Kwon N, Nordstrom B, et al. Burden of illness associated with eosinophilic granulomatosis with polyangiitis: a systematic literature review and meta-analysis. Clin Rheumatol 2021;40:4829-36. [Crossref] [PubMed]
- Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394:407-18. [Crossref] [PubMed]
- Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691-706. [Crossref] [PubMed]
- Long Z, Liu W, Qi J, et al. Mortality and Trend of Chronic Respiratory diseases in China from 1990 to 2019. Chinese Journal of Epidemiology 2022;43:14-21. [Crossref] [PubMed]
- Ma Y, Zhang W, Yu B, et al. Prevalence of allergic bronchopulmonary aspergillosis in Chinese patients with bronchial asthma. Chinese Journal of Tuberculosis and Respiratory Diseases 2011;34:909-13.
- Gómez de la Fuente E, Alobid I, Ojanguren I, et al. Addressing the unmet needs in patients with type 2 inflammatory diseases: when quality of life can make a difference. Front Allergy 2023;4:1296894. [Crossref] [PubMed]
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. Chinese Journal of Evidence-based Medicine 2009;9:8-11.
- Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. [Crossref] [PubMed]
- Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 2015;135:626-35. [Crossref] [PubMed]
- Chinese Society of Allergology. Wang L, Sun Y. Expert consensus on the mechanism and targeted therapy of type 2 inflammatory diseases. National Medical Journal of China 2022;102:3349-73.
- Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol 2015;36:189-95. [Crossref] [PubMed]
- Robinson D, Hamid Q, Bentley A, et al. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol 1993;92:313-24. [Crossref] [PubMed]
- Peters MC, Mekonnen ZK, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol 2014;133:388-94. [Crossref] [PubMed]
- Ying S, O'Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol 2008;181:2790-8. [Crossref] [PubMed]
- Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013;123:3967-82. [Crossref] [PubMed]
- Kim SW, Rhee CK, Kim KU, et al. Factors associated with plasma IL-33 levels in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2017;12:395-402. [Crossref] [PubMed]
- Jakiela B, Szczeklik W, Plutecka H, et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg-Strauss syndrome patients. Rheumatology (Oxford) 2012;51:1887-93. [Crossref] [PubMed]
- Dallos T, Heiland GR, Strehl J, et al. CCL17/thymus and activation-related chemokine in Churg-Strauss syndrome. Arthritis Rheum 2010;62:3496-503. [Crossref] [PubMed]
- Lloyd CM, Snelgrove RJ. Type 2 immunity: Expanding our view. Sci Immunol 2018;3:eaat1604.
- Halim TY, Steer CA, Mathä L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014;40:425-35. [Crossref] [PubMed]
- Mortaz E, Amani S, Mumby S, et al. Role of Mast Cells and Type 2 Innate Lymphoid (ILC2) Cells in Lung Transplantation. J Immunol Res 2018;2018:2785971. [Crossref] [PubMed]
- Jain A, Pasare C. Innate Control of Adaptive Immunity: Beyond the Three-Signal Paradigm. J Immunol 2017;198:3791-800. [Crossref] [PubMed]
- Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 2013;1:e24333. [Crossref] [PubMed]
- Sugita K, Steer CA, Martinez-Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol 2018;141:300-310.e11. [Crossref] [PubMed]
- McBrien CN, Menzies-Gow A. The Biology of Eosinophils and Their Role in Asthma. Front Med (Lausanne) 2017;4:93. [Crossref] [PubMed]
- Nakagome K, Nagata M. The Possible Roles of IL-4/IL-13 in the Development of Eosinophil-Predominant Severe Asthma. Biomolecules 2024;14:546. [Crossref] [PubMed]
- Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest 2018;128:997-1009. [Crossref] [PubMed]
- Dunican EM, Elicker BM, Henry T, et al. Mucus Plugs and Emphysema in the Pathophysiology of Airflow Obstruction and Hypoxemia in Smokers. Am J Respir Crit Care Med 2021;203:957-68. [Crossref] [PubMed]
- Bonser LR, Zlock L, Finkbeiner W, et al. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest 2016;126:2367-71. [Crossref] [PubMed]
- Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809-21. [Crossref] [PubMed]
- Malavia NK, Mih JD, Raub CB, et al. IL-13 induces a bronchial epithelial phenotype that is profibrotic. Respir Res 2008;9:27. [Crossref] [PubMed]
- Ohno I, Nitta Y, Yamauchi K, et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol 1996;15:404-9. [Crossref] [PubMed]
- Halwani R, Vazquez-Tello A, Sumi Y, et al. Eosinophils induce airway smooth muscle cell proliferation. J Clin Immunol 2013;33:595-604. [Crossref] [PubMed]
- Pégorier S, Wagner LA, Gleich GJ, et al. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol 2006;177:4861-9. [Crossref] [PubMed]
- Manson ML, Säfholm J, James A, et al. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J Allergy Clin Immunol 2020;145:808-817.e2. [Crossref] [PubMed]
- Xiong DJP, Martin JG, Lauzon AM. Airway smooth muscle function in asthma. Front Physiol 2022;13:993406. [Crossref] [PubMed]
- Han L, Limjunyawong N, Ru F, et al. Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nat Neurosci 2018;21:324-8. [Crossref] [PubMed]
- Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009;139:1353-65. [Crossref] [PubMed]
- Talbot S, Abdulnour RE, Burkett PR, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 2015;87:341-54. [Crossref] [PubMed]
- Satia I, Watson R, Scime T, et al. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J Allergy Clin Immunol 2019;144:788-795.e1. [Crossref] [PubMed]
- Global Strategy for Asthma Management and Prevention, 2024. Updated May 2024. Available online: www.ginasthma.org
- Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol 2012;129:S9-23. [Crossref] [PubMed]
- Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 1989;320:271-7. [Crossref] [PubMed]
- Ahmad Al Obaidi AH, Mohamed Al Samarai AG, Yahya Al Samarai AK, et al. The predictive value of IgE as biomarker in asthma. J Asthma 2008;45:654-63. [Crossref] [PubMed]
- Lommatzsch M, Speer T, Herr C, et al. IgE is associated with exacerbations and lung function decline in COPD. Respir Res 2022;23:1. [Crossref] [PubMed]
- Fitzsimmons CM, Falcone FH, Dunne DW. Helminth Allergens, Parasite-Specific IgE, and Its Protective Role in Human Immunity. Front Immunol 2014;5:61. [Crossref] [PubMed]
- Nafría Jiménez B, Oliveros Conejero R. IgE multiple myeloma: detection and follow-up. Adv Lab Med 2022;3:79-90. [Crossref] [PubMed]
- Cowan DC, Taylor DR, Peterson LE, et al. Biomarker-based asthma phenotypes of corticosteroid response. J Allergy Clin Immunol 2015;135:877-883.e1. [Crossref] [PubMed]
- ten Brinke A, Zwinderman AH, Sterk PJ, et al. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med 2001;164:744-8. [Crossref] [PubMed]
- Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014;44:1697-700. [Crossref] [PubMed]
- Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017;5:956-67. [Crossref] [PubMed]
- Ortega H, Katz L, Gunsoy N, et al. Blood eosinophil counts predict treatment response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2015;136:825-6. [Crossref] [PubMed]
- Semprini R, Williams M, Semprini A, et al. Type 2 Biomarkers and Prediction of Future Exacerbations and Lung Function Decline in Adult Asthma. J Allergy Clin Immunol Pract 2018;6:1982-1988.e1. [Crossref] [PubMed]
- Backman H, Lindberg A, Hedman L, et al. FEV(1) decline in relation to blood eosinophils and neutrophils in a population-based asthma cohort. World Allergy Organ J 2020;13:100110. [Crossref] [PubMed]
- Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur Respir J 2023;61:2300239. [Crossref] [PubMed]
- Smith AD, Cowan JO, Brassett KP, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005;172:453-9. [Crossref] [PubMed]
- Kupczyk M, ten Brinke A, Sterk PJ, et al. Frequent exacerbators--a distinct phenotype of severe asthma. Clin Exp Allergy 2014;44:212-21. [Crossref] [PubMed]
- Maniscalco M, Fuschillo S, Mormile I, et al. Exhaled Nitric Oxide as Biomarker of Type 2 Diseases. Cells 2023;12:2518. [Crossref] [PubMed]
- Xu X, Zhou L, Tong Z. The Relationship of Fractional Exhaled Nitric Oxide in Patients with AECOPD. Int J Chron Obstruct Pulmon Dis 2023;18:3037-46. [Crossref] [PubMed]
- Alcázar-Navarrete B, Díaz-Lopez JM, García-Flores P, et al. T2 Biomarkers as Predictors of Exacerbations of Chronic Obstructive Pulmonary Disease. Arch Bronconeumol 2022;58:595-600. [Crossref] [PubMed]
- Pereira Santos MC, Campos Melo A, Caetano A, et al. Longitudinal study of the expression of FcεRI and IgE on basophils and dendritic cells in association with basophil function in two patients with severe allergic asthma treated with Omalizumab. Eur Ann Allergy Clin Immunol 2015;47:38-40.
- Pelaia C, Vatrella A, Gallelli L, et al. Dupilumab for the treatment of asthma. Expert Opin Biol Ther 2017;17:1565-72. [Crossref] [PubMed]
- Pelaia C, Vatrella A, Busceti MT, et al. Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des Devel Ther 2017;11:3137-44. [Crossref] [PubMed]
- Pelaia C, Vatrella A, Bruni A, et al. Benralizumab in the treatment of severe asthma: design, development and potential place in therapy. Drug Des Devel Ther 2018;12:619-28. [Crossref] [PubMed]
- Marone G, Spadaro G, Braile M, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs 2019;28:931-40. [Crossref] [PubMed]
- Kuruvilla ME, Lee FE, Lee GB. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin Rev Allergy Immunol 2019;56:219-33. [Crossref] [PubMed]
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388-95. [Crossref] [PubMed]
- Schleich F, Brusselle G, Louis R, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med 2014;108:1723-32. [Crossref] [PubMed]
- Deng Z, Jin M, Ou C, et al. Eligibility of C-BIOPRED severe asthma cohort for type-2 biologic therapies. Chin Med J (Engl) 2023;136:230-2. [Crossref] [PubMed]
- Li J, Huang Y, Lin X, et al. Influence of degree of specific allergic sensitivity on severity of rhinitis and asthma in Chinese allergic patients. Respir Res 2011;12:95. [Crossref] [PubMed]
- Chung KF, Dixey P, Abubakar-Waziri H, et al. Characteristics, phenotypes, mechanisms and management of severe asthma. Chin Med J (Engl) 2022;135:1141-55. [Crossref] [PubMed]
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. [Crossref] [PubMed]
- Busse WW, Kraft M, Rabe KF, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J 2021;58:2003393. [Crossref] [PubMed]
- Asthma group of Chinese Throacic Society. Guidelines for bronchial asthma prevent and management(2020 edition) Asthma group of Chinese Throacic Society. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:1023-48. [Crossref] [PubMed]
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001;108:184-90. [Crossref] [PubMed]
- Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001;18:254-61. [Crossref] [PubMed]
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16. [Crossref] [PubMed]
- Lanier B, Bridges T, Kulus M, et al. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol 2009;124:1210-6. [Crossref] [PubMed]
- Li J, Kang J, Wang C, et al. Omalizumab Improves Quality of Life and Asthma Control in Chinese Patients With Moderate to Severe Asthma: A Randomized Phase III Study. Allergy Asthma Immunol Res 2016;8:319-28. [Crossref] [PubMed]
- Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med 2018;378:2486-96. [Crossref] [PubMed]
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med 2018;378:2475-85. [Crossref] [PubMed]
- Bacharier LB, Maspero JF, Katelaris CH, et al. Dupilumab in Children with Uncontrolled Moderate-to-Severe Asthma. N Engl J Med 2021;385:2230-40. [Crossref] [PubMed]
- Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022;10:11-25. [Crossref] [PubMed]
- Zhang Q, Zhong N, Fang H, et al. Efficacy and Safety of Dupilumab in Patients from China with Persistent Asthma: A Subgroup Analysis of a Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Phase 3 Study. Am J Respir Crit Care Med 2023;207:A4768.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198-207. [Crossref] [PubMed]
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014;371:1189-97. [Crossref] [PubMed]
- Khurana S, Brusselle GG, Bel EH, et al. Long-term Safety and Clinical Benefit of Mepolizumab in Patients With the Most Severe Eosinophilic Asthma: The COSMEX Study. Clin Ther 2019;41:2041-2056.e5. [Crossref] [PubMed]
- Chen R, Wei L, Dai Y, et al. Efficacy and safety of mepolizumab in a Chinese population with severe asthma: a phase III, randomised, double-blind, placebo-controlled trial. ERJ Open Res 2024;10:00750-2023. [Crossref] [PubMed]
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128-41. [Crossref] [PubMed]
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract 2021;9:4381-4392.e4. [Crossref] [PubMed]
- Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med 2021;9:260-74. [Crossref] [PubMed]
- Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med 2022;10:47-58. [Crossref] [PubMed]
- Lai K, Sun D, Dai R, et al. Benralizumab efficacy and safety in severe asthma: A randomized trial in Asia. Respir Med 2024; Epub ahead of print. [Crossref]
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med 2021;384:1800-9. [Crossref] [PubMed]
- Wechsler ME, Menzies-Gow A, Brightling CE, et al. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir Med 2022;10:650-60. [Crossref] [PubMed]
- Menzies-Gow A, Wechsler ME, Brightling CE, et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomised, placebo-controlled extension study. Lancet Respir Med 2023;11:425-38. [Crossref] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Evidence-based strategy document for COPD diagnosis, management, and prevention, with citations from the scientific literature. 2024 REPORT. 2024 GOLD Report - Global Initiative for Chronic Obstructive Lung Disease – GOLD. Available online: https://goldcopd.org/2024-gold-report/#:~:text=Evidence-based%20strategy%20document%20for%20COPD%20diagnosis%2C%20management%2C%20and,scientific%20literature.%20View%20the%202024%20Summary%20of%20Changes
- Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. BMJ 2022;378:e069679. [Crossref] [PubMed]
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706-17.
- Expert Consensus on Immunomodulatory Therapy for Chronic Obstructive Pulmonary Disease (COPD) Writing Group. Expert consensus on immunomodulatory therapy for chronic obstructive pulmonary disease. Chinese General Practice 2022;25:2947-59.
- Halpin DMG, de Jong HJI, Carter V, et al. Distribution, Temporal Stability and Appropriateness of Therapy of Patients With COPD in the UK in Relation to GOLD 2019. EClinicalMedicine 2019;14:32-41. [Crossref] [PubMed]
- Lange P, Ahmed E, Lahmar ZM, et al. Natural history and mechanisms of COPD. Respirology 2021;26:298-321. [Crossref] [PubMed]
- Sharma V, Ricketts HC, Steffensen F, et al. Obesity affects type 2 biomarker levels in asthma. J Asthma 2023;60:385-92. [Crossref] [PubMed]
- Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J 2017;50:1701162. [Crossref] [PubMed]
- Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N Engl J Med 2023;389:205-14. [Crossref] [PubMed]
- Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N Engl J Med 2024;390:2274-83. [Crossref] [PubMed]
- Shi X, Tan CF, Chen XY, et al. Efficacy of dupilumab in the treatment of COPD with type 2 inflammation: A real-world study. Allergy Medicine 2024;2:100015.
- Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the Prevention of COPD Exacerbations. N Engl J Med 2019;381:1023-34. [Crossref] [PubMed]
- Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N Engl J Med 2017;377:1613-29. [Crossref] [PubMed]
- Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021;9:1288-98. [Crossref] [PubMed]
- Yousuf AJ, Mohammed S, Carr L, et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial. Lancet Respir Med 2022;10:469-77. [Crossref] [PubMed]
- Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73. [Crossref] [PubMed]
- Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol 2021;147:1261-1268.e5. [Crossref] [PubMed]
- Zeng Y, Xue X, Cai H, et al. Clinical Characteristics and Prognosis of Allergic Bronchopulmonary Aspergillosis: A Retrospective Cohort Study. J Asthma Allergy 2022;15:53-62. [Crossref] [PubMed]
- Dietschmann A, Schruefer S, Krappmann S, et al. Th2 cells promote eosinophil-independent pathology in a murine model of allergic bronchopulmonary aspergillosis. Eur J Immunol 2020;50:1044-56. [Crossref] [PubMed]
- Agarwal R, Muthu V, Sehgal IS, et al. Allergic Bronchopulmonary Aspergillosis. Clin Chest Med 2022;43:99-125. [Crossref] [PubMed]
- Chen H, Zhang X, Zhu L, et al. Clinical and immunological characteristics of Aspergillus fumigatus-sensitized asthma and allergic bronchopulmonary aspergillosis. Front Immunol 2022;13:939127. [Crossref] [PubMed]
- Chen X, Zhi H, Wang X, et al. Efficacy of Biologics in Patients with Allergic Bronchopulmonary Aspergillosis: A Systematic Review and Meta-Analysis. Lung 2024;202:367-83. [Crossref] [PubMed]
- Voskamp AL, Gillman A, Symons K, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2015;3:192-9. [Crossref] [PubMed]
- Multidisciplinary Expert Consensus Writing Group on the Diagnosis and Treatment of Eosinophilic Granulomatis with Poyangiitis. Multidisciplinary expert consensus on the diagnosis and treatment of eosinophilic granulomatosis with polyangiitis. Chinese Journal of Tuberculosis and Respiration 2018;41:8.
- Kouverianos I, Angelopoulos A, Daoussis D. The role of anti-eosinophilic therapies in eosinophilic granulomatosis with polyangiitis: a systematic review. Rheumatol Int 2023;43:1245-52. [Crossref] [PubMed]
- Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol 1986;39:177-253. [Crossref] [PubMed]
- Milne ME, Kimball J, Tarrant TK, et al. The Role of T Helper Type 2 (Th2) Cytokines in the Pathogenesis of Eosinophilic Granulomatosis with Polyangiitis (eGPA): an Illustrative Case and Discussion. Curr Allergy Asthma Rep 2022;22:141-50. [Crossref] [PubMed]
- Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol 2019;143:2170-7. [Crossref] [PubMed]
- Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017;376:1921-32. [Crossref] [PubMed]
- Bettiol A, Urban ML, Bello F, et al. Sequential rituximab and mepolizumab in eosinophilic granulomatosis with polyangiitis (EGPA): a European multicentre observational study. Ann Rheum Dis 2022;81:1769-72. [Crossref] [PubMed]
- Bettiol A, Urban ML, Padoan R, et al. Benralizumab for eosinophilic granulomatosis with polyangiitis: a retrospective, multicentre, cohort study. Lancet Rheumatol 2023;5:e707-15. [Crossref] [PubMed]
- Wechsler ME, Nair P, Terrier B, et al. Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2024;390:911-21. [Crossref] [PubMed]
- Cai C. Unmasking nonasthmatic eosinophilic bronchitis as an important cause of chronic cough. Medical Journal of Chinese People's Liberation Army 2014;39:365-8.
- Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med 1999;160:406-10. [Crossref] [PubMed]
- Lai K, Chen R, Peng W, et al. Non-asthmatic eosinophilic bronchitis and its relationship with asthma. Pulm Pharmacol Ther 2017;47:66-71. [Crossref] [PubMed]
- Zhan C, Xu R, Li B, et al. Eosinophil Progenitors in Patients With Non-Asthmatic Eosinophilic Bronchitis, Eosinophilic Asthma, and Normal Controls. Front Immunol 2022;13:737968. [Crossref] [PubMed]
- Zhan C, Liu J, Li B, et al. Group 2 innate lymphoid cells in patients with nonasthmatic eosinophilic bronchitis. Allergy 2022;77:649-52. [Crossref] [PubMed]
- Zhan W, Lai K. Research progress of eosinophilic bronchitis. Chinese Journal of Tuberculosis and Respiration 2023;46:192-6. [Crossref] [PubMed]
- Liu X, Wang X, Yao X, et al. Value of Exhaled Nitric Oxide and FEF(25-75) in Identifying Factors Associated With Chronic Cough in Allergic Rhinitis. Allergy Asthma Immunol Res 2019;11:830-45. [Crossref] [PubMed]
- Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy 2005;35:598-601. [Crossref] [PubMed]
- Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol 2008;147:1-5. [Crossref] [PubMed]
- Brightling CE, Woltmann G, Wardlaw AJ, et al. Development of irreversible airflow obstruction in a patient with eosinophilic bronchitis without asthma. Eur Respir J 1999;14:1228-30. [Crossref] [PubMed]
- Cockcroft DW. Eosinophilic bronchitis as a cause of cough. Chest 2000;118:277. [Crossref] [PubMed]
- Mepolizumab: 240563, anti-IL-5 monoclonal antibody - GlaxoSmithKline, anti-interleukin-5 monoclonal antibody - GlaxoSmithKline, SB 240563. Drugs R D 2008;9:125-30. [Crossref] [PubMed]
- Suzuki Y, Suda T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int 2019;68:413-9. [Crossref] [PubMed]
- Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA 2004;292:2997-3005. [Crossref] [PubMed]
- De Giacomi F, Vassallo R, Yi ES, et al. Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management. Am J Respir Crit Care Med 2018;197:728-36. [Crossref] [PubMed]
- Yang J, Cai H. Research Progress on Acute Eosinophilic Pneumonia. Chinese Journal of Respiratory and Critical Care 2015;14:4.
- Allen J, Wert M. Eosinophilic Pneumonias. J Allergy Clin Immunol Pract 2018;6:1455-61. [Crossref] [PubMed]
- Zhang X, Gong H, Gao J. Clinical Analysis of 40 Patients with Eosinophilic Lung Diseases in Peking Union Medical College Hospital. Acta Academiae Medicinae Sinicae 2018;40:170-7. [Crossref] [PubMed]
- Lin RY, Santiago TP, Patel NM. Favorable response to asthma-dosed subcutaneous mepolizumab in eosinophilic pneumonia. J Asthma 2019;56:1193-7. [Crossref] [PubMed]
- To M, Kono Y, Yamawaki S, et al. A case of chronic eosinophilic pneumonia successfully treated with mepolizumab. J Allergy Clin Immunol Pract 2018;6:1746-1748.e1. [Crossref] [PubMed]
- Bronchiectasis Expert Consensus Writing Collaboration Group. Expert consensus on the diagnosis and treatment of bronchiectasis in adults in China. Chinese Journal of Tuberculosis and Respiration 2021;44:11.
- Tsikrika S, Dimakou K, Papaioannou AI, et al. The role of non-invasive modalities for assessing inflammation in patients with non-cystic fibrosis bronchiectasis. Cytokine 2017;99:281-6. [Crossref] [PubMed]
- Shoemark A, Shteinberg M, De Soyza A, et al. Characterization of Eosinophilic Bronchiectasis: A European Multicohort Study. Am J Respir Crit Care Med 2022;205:894-902. [Crossref] [PubMed]
- Oriano M, Gramegna A, Amati F, et al. T2-High Endotype and Response to Biological Treatments in Patients with Bronchiectasis. Biomedicines 2021;9:772. [Crossref] [PubMed]
- Agaronyan K, Sharma L, Vaidyanathan B, et al. Tissue remodeling by an opportunistic pathogen triggers allergic inflammation. Immunity 2022;55:895-911.e10. [Crossref] [PubMed]
- Guan WJ, Oscullo G, He MZ, et al. Significance and Potential Role of Eosinophils in Non-Cystic Fibrosis Bronchiectasis. J Allergy Clin Immunol Pract 2023;11:1089-99. [Crossref] [PubMed]
- Crimi C, Campisi R, Cacopardo G, et al. Real-life effectiveness of mepolizumab in patients with severe refractory eosinophilic asthma and multiple comorbidities. World Allergy Organ J 2020;13:100462. [Crossref] [PubMed]
- Kudlaty E, Patel GB, Prickett ML, et al. Efficacy of type 2-targeted biologics in patients with asthma and bronchiectasis. Ann Allergy Asthma Immunol 2021;126:302-4. [Crossref] [PubMed]
- Record History | ver. 5: 2021-11-16 | NCT05006573 | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05006573
- Maspero J, Adir Y, Al-Ahmad M, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res 2022;8:00576-2021. [Crossref] [PubMed]
- ten Brinke A, Grootendorst DC, Schmidt JT, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol 2002;109:621-6. [Crossref] [PubMed]
- Yawn BP, Yunginger JW, Wollan PC, et al. Allergic rhinitis in Rochester, Minnesota residents with asthma: frequency and impact on health care charges. J Allergy Clin Immunol 1999;103:54-9. [Crossref] [PubMed]
- Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology 2019;57:32-42. [Crossref] [PubMed]
- Peters AT, Bengtson LGS, Chung Y, et al. Clinical and economic burden of chronic rhinosinusitis with nasal polyposis: A U.S. administrative claims analysis. Allergy Asthma Proc 2022;43:435-45. [Crossref] [PubMed]
- McDonald VM, Osadnik CR, Gibson PG. Treatable traits in acute exacerbations of chronic airway diseases. Chron Respir Dis 2019;16:1479973119867954. [Crossref] [PubMed]
- Armengot-Carceller M, Gómez-Gómez MJ, García-Navalón C, et al. Effects of Omalizumab Treatment in Patients With Recalcitrant Nasal Polyposis and Mild Asthma: A Multicenter Retrospective Study. Am J Rhinol Allergy 2021;35:516-24. [Crossref] [PubMed]
- Gallo S, Castelnuovo P, Spirito L, et al. Mepolizumab Improves Outcomes of Chronic Rhinosinusitis with Nasal Polyps in Severe Asthmatic Patients: A Multicentric Real-Life Study. J Pers Med 2022;12:1304. [Crossref] [PubMed]
- Detoraki A, Tremante E, D'Amato M, et al. Mepolizumab improves sino-nasal symptoms and asthma control in severe eosinophilic asthma patients with chronic rhinosinusitis and nasal polyps: a 12-month real-life study. Ther Adv Respir Dis 2021;15:17534666211009398. [Crossref] [PubMed]
- Domínguez-Sosa MS, Cabrera-Ramírez MS, Marrero-Ramos MDC, et al. Efficacy of dupilumab on chronic rhinosinusitis with nasal polyps and concomitant asthma in biologic-naive and biologic-pretreated patients. Ann Med 2024;56:2411018. [Crossref] [PubMed]
- Nolasco S, Crimi C, Pelaia C, et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with and without Chronic Rhinosinusitis with Nasal Polyps: A Real-World Multicenter Study. J Allergy Clin Immunol Pract 2021;9:4371-4380.e4. [Crossref] [PubMed]
- Canonica GW, Bourdin A, Peters AT, et al. Dupilumab Demonstrates Rapid Onset of Response Across Three Type 2 Inflammatory Diseases. J Allergy Clin Immunol Pract 2022;10:1515-26. [Crossref] [PubMed]
- Busse WW, Maspero JF, Lu Y, et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol 2020;125:565-576.e1. [Crossref] [PubMed]
- Isoyama S, Ishikawa N, Hamai K, et al. Efficacy of mepolizumab in elderly patients with severe asthma and overlapping COPD in real-world settings: A retrospective observational study. Respir Investig 2021;59:478-86. [Crossref] [PubMed]
- Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med 2022;387:2317-30. [Crossref] [PubMed]
- Senna G, Micheletto C, Piacentini G, et al. Multidisciplinary management of type 2 inflammatory diseases. Multidiscip Respir Med 2022;17:813. [Crossref] [PubMed]
- Vassilopoulou E, Skypala I, Feketea G, et al. A multi-disciplinary approach to the diagnosis and management of allergic diseases: An EAACI Task Force. Pediatr Allergy Immunol 2022;33:e13692. [Crossref] [PubMed]
- Shiobara T, Chibana K, Watanabe T, et al. Dipeptidyl peptidase-4 is highly expressed in bronchial epithelial cells of untreated asthma and it increases cell proliferation along with fibronectin production in airway constitutive cells. Respir Res 2016;17:28. [Crossref] [PubMed]
- Malinovschi A, Rydell N, Fujisawa T, et al. Clinical Potential of Eosinophil-Derived Neurotoxin in Asthma Management. J Allergy Clin Immunol Pract 2023;11:750-61. [Crossref] [PubMed]
- Pavord ID, Afzalnia S, Menzies-Gow A, et al. The current and future role of biomarkers in type 2 cytokine-mediated asthma management. Clin Exp Allergy 2017;47:148-60. [Crossref] [PubMed]
- Ogulur I, Pat Y, Ardicli O, et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021;76:3659-86. [Crossref] [PubMed]
- An J, Lee JH, Won HK, et al. Clinical significance of serum MRGPRX2 as a new biomarker in allergic asthma. Allergy 2020;75:959-62. [Crossref] [PubMed]
- Peters MC, Nguyen ML, Dunican EM. Biomarkers of Airway Type-2 Inflammation and Integrating Complex Phenotypes to Endotypes in Asthma. Curr Allergy Asthma Rep 2016;16:71. [Crossref] [PubMed]
- Rogliani P, Manzetti GM, Bettin FR, et al. Investigational thymic stromal lymphopoietin inhibitors for the treatment of asthma: a systematic review. Expert Opin Investig Drugs 2024;33:39-49. [Crossref] [PubMed]
- Santus P, Saad M, Damiani G, et al. Current and future targeted therapies for severe asthma: Managing treatment with biologics based on phenotypes and biomarkers. Pharmacol Res 2019;146:104296. [Crossref] [PubMed]
- Matucci A, Vivarelli E, Nencini F, et al. Strategies Targeting Type 2 Inflammation: From Monoclonal Antibodies to JAK-Inhibitors. Biomedicines 2021;9:1497. [Crossref] [PubMed]
- Zarrin AA, Bao K, Lupardus P, et al. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discov 2021;20:39-63. [Crossref] [PubMed]
- Song R, Zhang H, Liang Z. Research progress in OX40/OX40L in allergic diseases. Int Forum Allergy Rhinol 2024;14:1921-8. [Crossref] [PubMed]


